Abstract
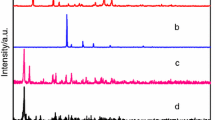
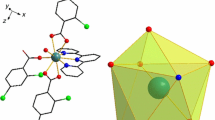
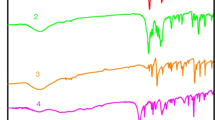
Two novel binuclear lanthanide complexes [Tb(2,3-DMOBA)3(5,5′-DM-2,2′-bipy)]2·C2H5OH (1) and [Dy(2,3-DMOBA)3(5,5′-DM-2,2′-bipy)]2·C2H5OH (2) (2,3-DMOBA = 2,3-dimethoxybenzoate, 5,5′-DM-2,2′-bipy = 5,5′-dimety-2,2′-bipyridine) have been successfully synthesized and structurally validated by single-crystal diffraction. The results of single-crystal analyses indicate the complexes contains one free ethanol molecule, and each center Ln(III) is nine-coordinated, exhibiting a distorted monocapped square anti-prismatic coordination geometry. The two center Ln(III) are bound by four 2,3-DMOBA ligands, two of which are bridging bidentate and the other two are bridging–chelating. The adjacent binuclear complexes can form 1D supramolecular structure by a pair of alternating identical C–H···O hydrogen bonding interactions, which further form 2D sheet structures. The thermal behavior of these complexes is investigated by TG-DSC/FTIR. What is more, the heat capacities of the complexes 1–2 are measured by DSC at 259.15–346.15 K, and the result indicates that the heat capacity values of the complexes gradually increased with the increase in temperature. In addition, the thermodynamic functions values (HT − H298.15K) and (ST − S298.15K) of the complexes 1–2 are calculated according to the fitted polynomial equations and the thermodynamic equation. The luminescence property of complex 1 is studied.









Similar content being viewed by others
References
Maji S, Viswanathan KS. Sensitization of uranium fluorescence using 2,6-pyridinedicarboxylic acid: application for the determination of uranium in the presence of lanthanides. J Lumin. 2009;129(11):1242–8. https://doi.org/10.1016/j.jlumin.2009.06.018.
Safarbali R, Yaftian MR, Zamani A. Solvent extraction-separation of La(III), Eu(III) and Er(III) ions from aqueous chloride medium using carbamoyl-carboxylic acid extractants. J Rare Earths. 2016;34(1):91–8. https://doi.org/10.1016/s1002-0721(14)60583-4.
Deng ZP, Kang W, Huo LH, Zhao H, Gao S. Rare-earth organic frameworks involving three types of architecture tuned by the lanthanide contraction effect: hydrothermal syntheses, structures and luminescence. Dalton Trans. 2010;39(27):6276–84. https://doi.org/10.1039/c0dt00031k.
Lv YG, Zhang JC, Cao WL, Fu YL. Enhanced luminescence of novel rare earth complexes Eu(3,5-DNBA)3Phen in nano-TiO2. Spectrochim Acta A Mol Biomol Spectrosc. 2009;72(1):22–5. https://doi.org/10.1016/j.saa.2008.07.003.
Hatanaka M, Wakabayashi T. Theoretical study of lanthanide-based in vivo luminescent probes for detecting hydrogen peroxide. J Comput Chem. 2019;40(2):500–6. https://doi.org/10.1002/jcc.25737.
Wang YW, Zhang YL, Dou W, Zhang AJ, Qin WW, Liu WS. Synthesis, radii dependent self-assembly crystal structures and luminescent properties of rare earth(III) complexes with a tripodal salicylic derivative. Dalton Trans. 2010;39(38):9013–21. https://doi.org/10.1039/c001780a.
Cagnin F, Davolos MR, Castellano EE. A polymeric europium complex with the ligand thiophene-2-carboxylic acid: synthesis, structural and spectroscopic characterization. Polyhedron. 2014;67:65–72. https://doi.org/10.1016/j.poly.2013.08.063.
Liu QY, Wang WF, Wang YL, Shan ZM, Wang MS, Tang J. Diversity of lanthanide(III)-organic extended frameworks with a 4,8-disulfonyl-2,6-naphthalenedicarboxylic acid ligand: syntheses, structures, and magnetic and luminescent properties. Inorg Chem. 2012;51(4):2381–92. https://doi.org/10.1021/ic2023727.
Yuan R, Chen C, Zhang N. Targeted highly-thermostable metal-organic frameworks directed by imidazole: syntheses, structures, thermal behaviors and luminescent properties. J Inorg Organomet Polym. 2012;22(2):507–13. https://doi.org/10.1007/s10904-011-9649-5.
Feng R, Jiang FL, Wu MY, Chen L, Yan CF, Hong MC. Structures and photoluminescent properties of the lanthanide coordination complexes with hydroxyquinoline carboxylate ligands. Cryst Growth Design. 2010;10(5):2306–13. https://doi.org/10.1021/cg100026d.
Zhang HB, Peng Y, Shan XC, Tian CB, Ping L, Du SW. Lanthanide metal organic frameworks based on octahedral secondary building units: structural, luminescent, and magnetic properties. Inorg Chem Commun. 2011;14(7):1165–9. https://doi.org/10.1016/j.inoche.2011.04.014.
Peng JB, Kong XJ, Ren YP, Long LS, Huang RB, Zheng LS. Trigonal bipyramidal Dy5 cluster exhibiting slow magnetic relaxation. Inorg Chem. 2012;51(4):2186–90. https://doi.org/10.1021/ic202147h.
Bai ZS, Xu J, Okamura TA, Chen MS, Sun WY, Ueyama N. Novel dense organic-lanthanide hybrid architectures: syntheses, structures and magnetic properties. Dalton Trans. 2009;14:2528–39. https://doi.org/10.1039/b816205k.
Kofod N, Arppe-Tabbara R, Sorensen TJ. Electronic energy levels of dysprosium(III) ions in solution. assigning the emitting state and the intraconfigurational 4f–4f transitions in the vis-NIR region and photophysical characterization of Dy(III) in water, methanol, and dimethyl sulfoxide. J Phys Chem A. 2019;123(13):2734–44. https://doi.org/10.1021/acs.jpca.8b12034.
Soek RN, Ferreira CM, Santana FS, Hughes DL, Poneti G, Ribeiro RR, et al. Structure and magnetic properties of two new lanthanide complexes with the 1-((E)-2-pyridinylmethylidene)semicarbazone ligand. J Mol Struct. 2019;1184:254–61. https://doi.org/10.1016/j.molstruc.2019.02.036.
Matsushita AFY, Pais A, Valente AJM. Energy transfer and multicolour tunable emission of Eu, Tb(PSA)Phen composites. Colloids Surf A. 2019;569:93–101. https://doi.org/10.1016/j.colsurfa.2019.02.049.
Su SQ, Wang S, Song XZ, Song SY, Qin C, Zhu M, et al. Syntheses, structures, photoluminescence, and magnetic properties of (3,6)-and 4-connected lanthanide metal-organic frameworks with a semirigid tricarboxylate ligand. Dalton Trans. 2012;41(16):4772–9. https://doi.org/10.1039/c2dt12346k.
Qi XX, Ren N, Xu SL, Zhang JJ, Zong GC, Gao J, et al. Lanthanide complexes with 3,4,5-triethoxybenzoic acid and 1,10-phenanthroline: synthesis, crystal structures, thermal decomposition mechanism and phase transformation kinetics. RSC Adv. 2015;5(12):9261–71. https://doi.org/10.1039/c4ra12063a.
Wu XH, He SM, Ren N, Zhang JJ. Crystal structures, thermal decomposition kinetics and thermodynamic properties of lanthanide Ho(III) complex with 2,5-dichlorobenzoic acid and 5,5′-dimethyl-2,2′-bipyridine. Sci Sin Chim. 2019;49:978–89. https://doi.org/10.1360/N032018-00222.
Ren N, Wang F, Zhang JJ, Zheng XF. Progress in thermal analysis kinetics. Acta Phys Chim Sin. 2019;35:0001–9. https://doi.org/10.3866/pku.whxb201905062.
Shen PP, Zhu MM, Ren N, Zhang JJ, Wang SP. Four novel lanthanide complexes with 4-ethylbenzoic acid and 5,5′-dimethy-2,2′-bipyridine: structures, luminescent, thermal properties and bacteriostatic activities. Appl Organomet Chem. 2017;31(12):e3886. https://doi.org/10.1002/aoc.3886.
Melnikov P, Arkhangelsky IV, Nascimento VA, de Oliveira LCS, Silva AF, Zanoni LZ. Thermal properties of europium nitrate hexahydrate Eu(NO3)3•6H2O. J Therm Anal Calorim. 2016;128(3):1353–8. https://doi.org/10.1007/s10973-016-6047-9.
Yang HP, Yan R, Chen HP, Lee DH, Zheng CC. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86(12–13):1781–8. https://doi.org/10.1016/j.fuel.2006.12.013.
Wang Y, Shen PP, Ren N, Zhang JJ, Geng LN, Wang SP, et al. A series of lanthanide complexes with different N-donor ligands: synthesis, structures, thermal properties and luminescence behaviors. RSC Adv. 2016;6(75):70770–80. https://doi.org/10.1039/c6ra11393a.
Wu XH, Ren N, Zhang JJ, Wang DQ. Lanthanide complexes with 2-bromo-5-methoxybenzoic acid and 5,5′-dimethyl-2,2′-bipyridine: crystal structures, thermodynamic properties and luminescence behaviors. J Chem Thermodyn. 2018;123:99–106. https://doi.org/10.1016/j.jct.2018.04.002.
Gao HL, Jiang L, Liu S, Shen HY, Wang WM, Cui JZ. Multiple magnetic relaxation processes, magnetocaloric effect and fluorescence properties of rhombus-shaped tetranuclear rare earth complexes. Dalton Trans. 2016;45(1):253–64. https://doi.org/10.1039/c5dt03790e.
Sun CM, Shen J, Cui RW, Yuan FZ, Zhang H, Wu X. Silver nanoflowers-enhanced Tb(III)/La(III) co-luminescence for the sensitive detection of dopamine. Anal Bioanal Chem. 2019;411(7):1375–81. https://doi.org/10.1007/s00216-018-01568-2.
Liu YH, Kong WH, Yang ZH, Dai M, Shi L, Guo DC. Synthesis and fluorescence properties of Eu3+, Tb3+ complexes with Schiff base derivatives. J Fluoresc. 2016;26(2):567–76. https://doi.org/10.1007/s10895-015-1741-8.
Li HL, Liu YJ, Zheng R, Ma X, Chen LJ, Zhao JW. Syntheses, structures and fluorescence properties of three rare-earth containing docosatungstates. Spectrochim Acta Part A. 2017;176:114–22. https://doi.org/10.1016/j.saa.2017.01.016.
Zhu MM, Zhang Z, Ren N, Wang SP, Zhang JJ. Rare earth complexes with 3,4-dimethylbenzoic acid and 2,2:6′,2″-terpyridine: synthesis, crystal structures, luminescence and thermodynamic properties. Inorg Chim Acta. 2019;484:311–8. https://doi.org/10.1016/j.ica.2018.09.061.
Cunha CS, Köppen M, Terraschke H, Friedrichs G, Malta OL, Stock N, et al. Luminescence tuning and single-phase white light emitters based on rare earth ions doped into a bismuth coordination network. J Mater Chem C. 2018;6(46):12668–78. https://doi.org/10.1039/c8tc04442b.
Feng SY, Li WX, Zheng YS, Xin XD, Guo F, Cao XF. Syntheses and luminescence properties of two novel lanthanide (III) perchlorate complexes with phenacyl p-tolyl sulfoxide. J Lumin. 2015;162:92–6. https://doi.org/10.1016/j.jlumin.2015.01.057.
Acknowledgements
The research work was supported by the National Natural Science Foundation of China (No. 21803016).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, YY., Ren, N., He, SM. et al. Supramolecular structures, thermal decomposition mechanism and heat capacity of the novel binuclear Tb(III) and Dy(III) complexes with 2,3-dimethoxybenzoic acid and 5,5′-dimety-2,2′-bipyridine. J Therm Anal Calorim 140, 2435–2445 (2020). https://doi.org/10.1007/s10973-019-08944-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08944-9