Abstract
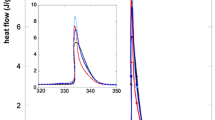
In this study, ZnO has been used as inorganic shell material (in situ synthetized) for the encapsulation of KNO3, an inorganic molten salt commonly used in concentrated solar plants applications. The thermal stability of microparticles encapsulated by using a solvothermal process has been optimized by adjusting the parameters affecting the properties of the microparticles, such as the core:shell ratio and the temperature during the microencapsulation process. The energy stored and released after each thermal cycle was evaluated by differential scanning calorimetry. Chemical composition of microparticles was evaluated by infrared spectroscopy and inductively coupled plasma spectroscopy, as well as morphology was characterized by scanning electron microscopy. Results have shown the solvothermal synthesis as a feasible process for the microencapsulation of molten salts by obtaining KNO3 particles covered by ZnO microcrystals. These particles have thermal energy storage and release capacities and temperatures similar to those of raw KNO3, being the temperature used during the solvothermal process the parameter determining the thermal stability of the microparticles, as demonstrated by carrying out durability tests through consecutive heating–cooling thermal cycles (250–400 °C).










Similar content being viewed by others

References
Nunes VMB, Queirós CS, Lourenço MJV, Santos FJV, Nieto de Castro CA. Molten salts as engineering fluids—A review. Part I. Molten alkali nitrates. Appl Energy. 2016;183:603–11.
Yu-ting Wu, Ying Li, Nan Ren, Chong-fang Ma. Improving the thermal properties of NaNO3–KNO3 for concentrating solar power by adding additives. Sol Energy Mater Sol Cells. 2017;160:263–8.
Raade JW, Padowitz D. Development of molten salt heat transfer fluid with low melting point and high thermal stability. J Sol Energy Eng. 2011;133(3):031013.
Vignarooban K, Xu X, Arvay A, Hsu K, Kannan AM. Heat transfer fluids for concentrating solar power systems—a review. Appl Energy. 2015;146:383–96.
Qiu S, Solomon L, Fang M. Study of material compatibility for a thermal energy storage system with phase change material. Energies. 2018;11:572.
Kuravi S, Trahan J, Yogi Goswami D, Rahman MM, Stefanakos EK. Thermal energy storage technologies and systems for concentrating solar power plants. Prog Energy Combust Sci. 2013;39:285–319.
Guillot S, Faik A, Rakhmatullin A, Lambert J, Veron E, Echegut P, Bessada C, Calvet N, Py X. Corrosion effects between molten salts and thermal storage material for concentrated solar power plants. Appl Energy. 2012;94:174–81.
Xu B, Li P, Chan Ch. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: a review to recent developments. Appl Energy. 2015;160:286–307.
Bilir L, İlken Z. Total solidification time of a liquid phase change material enclosed in cylindrical/spherical containers. Appl Therm Eng. 2005;25(10):1488–502.
Mathura A, Kasetty R, Oxley J, Mendez J, Nithyanandam K. Using encapsulated phase change salts for concentrated solar power plant. Energy Procedia. 2014;49:908–15.
US 2011/0259544. Encapsulated phase change apparatus for thermal energy storage.
Pitié F, Zhao CY, Cáceres G. Thermo-mechanical analysis of ceramic encapsulated phase-change-material (PCM) particles. Energy Environ Sci. 2011;4:2117–24.
Graham M, Shchukina E, Castro P, Shchukin D. Nanocapsules containing salt hydrate phase change materials for thermal energy storage. J Mater Chem A. 2016;4:16906–12.
US 2015/0284616. Encapsulation of thermal energy storage media.
Salunkhe PB, Shembekar PS. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew Sustain Energy Rev. 2012;16(8):5603–16.
Cáceres G, Fullenkamp K, Montané M, Naplocha K, Dmitruk A. Review encapsulated nitrates phase change material selection for use as thermal storage and heat transfer materials at high temperature in concentrated solar power plants. Energies. 2017;10(9):1318.
Milián YE, Gutiérrez A, Grágeda M, Ushak S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renew Sustain Energy Rev. 2017;73:983–99.
Platte D, Helbig U, Houbertz R, Sextl G. Microencapsulation of alkaline salt hydrate melts for phase change applications by surface thiol-michael addition polymerization. Macromol Mater Eng. 2013;298:67–77.
Shchukina EM, Graham M, Zheng Z, Shchukin DG. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem Soc Rev. 2018;47(11):4156–75.
Hong Y, Ding S, Wu W, Hu J, Voevodin AA, Gschwender L, Snyder E, Chow L, Su M. Enhancing heat capacity of colloidal suspension using nanoscale encapsulated phase-change materials for heat transfer. Appl Mater Interfaces. 2010;2:1685–91.
Sánchez L, Sánchez P, de Lucas A, Carmona M, Rodríguez JF. Microencapsulation of PCMs with a polystyrene shell. Colloid Polym Sci. 2007;285(12):1377–85.
Selçuk Mert M, Hande Mert H, Sert M. Microencapsulated oleic–capric acid/hexadecane mixture as phase change material for thermal energy storage. J Therm Anal Calorim. 2019;136(4):1551–61.
Su W, Zhou T, Li Y, Lv Y. Development of microencapsulated phase change material with poly (methyl methacrylate) shell for thermal energy storage. Energy Procedia. 2019;158:4483–8.
Maruoka N, Akiyama T. Thermal stress analysis of PCM encapsulation for heat recovery of high temperature waste heat. J Chem Eng Japan. 2003;36(7):794–8.
Nomura T, Sheng N, Zhu Ch, Saito G, Hanzaki D, Hiraki T, Akiyama T. Microencapsulated phase change materials with high heat capacity and high cyclic durability for high-temperature thermal energy storage and transportation. Appl Energy. 2017;188:9–18.
Tudor AI, Motoc AM, Ciobota CF, Ciobota DN, Piticescu RR, Romero-Sanchez MD. Solvothermal method as a green chemistry solution for microencapsulation of phase change materials for high temperature thermal energy storage. Manuf Rev. 2018;5(4):1–12.
Kenisarin MM. High-temperature phase change materials for thermal energy storage. Renew Sustain Energy Rev. 2010;14:955–70.
Ramakrishnan S, Sanjayan J, Wang X, Alam M, Wilson J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl Energy. 2015;157:85–94.
Mihaiu S, Madarász J, Pokol G, Szilágyi IM, Kaszás T, Mocioiu OC, Atkinson I, Toader A, Munteanu C, Marinescu VE, Zaharescu M. Thermal behaviour of ZnO precursor powders obtained from aqueous solutions. Rev Roum Chim. 2013;58(4–5):335–45.
Chouillet C, Krafft JM, Louis C, Lauron-Pernot H. Characterization of zinc hydroxynitrates by diffuse reflectance infrared spectroscopy-structural modifications during thermal treatment. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60(3):505–11.
Kee SY, Munusamy Y, Ong KS, Cornelis Metselaar HS, Chee SY, La KCh. Thermal performance study of composite phase change material with polyacrylicand conformal coating. Materials. 2017;10:873. https://doi.org/10.3390/ma10080873.
Sokolov PS, Baranov AN, Dobrokhotova ZhV, Solozhenkoa VL. Synthesis and thermal stability of cubic ZnO in the salt nanocomposites. Russ Chem Bull. 2010;59(2):325–8.
Nityashree N, Rajamathi M. Interstratified composite of the anionic clays, Zn5(OH)8(NO3)2·2H2O and Ni3Zn2(OH)8(NO3)2·2H2O, by delamination-costacking. J Phys Chem Solid. 2013;74(8):1164–8.
Nyquist RA, Kagel RO. Handbook of infrared and raman spectra of inorganic compounds and organic salts. New York and London: Elsevier Inc., Academic Press; 1971.
Newman SP, Jones W. Comparative study of some layered hydroxide salts containing exchangeable interlayer anions. J Solid State Chem. 1999;148:26–40.
Sari A, Kaygusuz K. Some fatty acids used for latent heat storage: thermal stability and corrosion of metals with respect to thermal cycling. Renew Energy. 2003;28:939–48.
Liu M, Bell S, Tay S, Will G, Saman W, Bruno F. Stability and corrosion testing of a high temperature phase change material for CSP applications. AIP Conf Proc. 2016;1734:050029.
Rathod M, Banerjee J. Thermal stability of phase change materials used in latent heat energy storage systems: a review. Renew Sustain Energy Rev. 2013;18:246–58.
Acknowledgements
The research leading to these results is based on the financial support from NASR, ENERHIGH project, under the Competitive Operational Programme 2014–2020. Contract 93/09.09.2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Romero-Sanchez, M.D., Piticescu, R.R., Motoc, A.M. et al. Preparation of microencapsulated KNO3 by solvothermal technology for thermal energy storage. J Therm Anal Calorim 138, 1979–1986 (2019). https://doi.org/10.1007/s10973-019-08825-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08825-1