Abstract
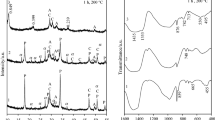
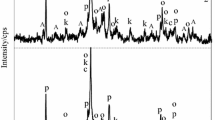
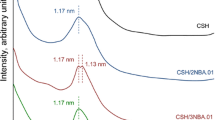
In this work, the adsorption capacity of Cu2+, Co2+ and Cr3+ by synthetic dibasic calcium silicate hydrate sample as well as its thermal stability was examined. The synthesis of dibasic calcium silicate hydrate was carried out in unstirred suspensions, when CaO/SiO2 molar ratio of primary mixture was equal to 1.5 and the duration of isothermal curing at 175 °C temperature was 16 h. Afterwards, metal ions were incorporated into the synthesis products; the experiments were carried out at 25 °C temperature by stirring 5 g of synthetic dibasic calcium silicate hydrate in 500 mL of Co(NO3)2·6H2O, Cr(NO3)2·9H2O or Cu(NO3)2·3H2O solutions containing 1 g of Co2+, Cr2+ or Cu2+ dm−3 for 30 min. It was obtained that after adsorption all metal ions (100 mg Me2+ g−1) were intercalated into the structure of synthesis products. In situ X-ray diffraction study on the thermal stability of synthesized compounds was performed in a modular temperature chamber from RT to 1000 °C temperature. The obtained results were confirmed by XRD, STA, FT-IR, AAS and SEM methods.








Similar content being viewed by others
References
Yuan H, Chini AR, Lu Y, Shen L. A dynamic model for assessing the effects of management strategies on the reduction of construction and demolition waste. Waste Manag (Oxford). 2012;32:521–31.
Bhatnagar A, Sillanpää M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—a review. Chem Eng J. 2010;157:277–96.
Generation and discharge of wastewater in volume. http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=env_ww_genv&lang=en. Accessed 30 Oct 2018
The United Nations world water development report. http://www.unwater.org/publications/world-water-development-report-2017/. Accessed 29 Oct 2018
Bhatnagar A, Sillanpää M. A review of emerging adsorbents for nitrate removal from water. Chem Eng J. 2011;168:493–504.
Soon AN, Hameed BH. Heterogeneous catalytic treatment of synthetic dyes in aqueous media using Fenton and photo-assisted Fenton process. Desalination. 2011;269:1–16.
Alver E, Metin AÜ. Anionic dye removal from aqueous solutions using modified zeolite: adsorption kinetics and isotherm studies. Chem Eng J. 2012;200–202:59–67.
Yuan H, Shen L. Trend of the research on construction and demolition waste management. Waste Manag. 2011;31:670–9.
Wang S, Sun H, Ang HM, Tadé MO. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem Eng J. 2013;226:336–47.
Sato T, Qadir M, Yamamoto S, Endo T, Zahoor A. Global, regional, and country level need for data on wastewater generation, treatment, and use. Agric Water Manag. 2013;130:1–13.
Chiang YW, Ghyselbrecht K, Santos RM, Meesschaert B, Martens JA. Synthesis of zeolitic-type adsorbent material from municipal solid waste incinerator bottom ash and its application in heavy metal adsorption. Catal Today. 2012;190:23–30.
Soto ML, Moure A, Domínguez H, Parajó JC. Recovery, concentration and purification of phenolic compounds by adsorption: a review. J Food Eng. 2011;105:1–27.
Caliskan N, Kul AR, Alkan S, Sogut EG, Alacabey İ. Adsorption of Zinc(II) on diatomite and manganese-oxide-modified diatomite: a kinetic and equilibrium study. J Hazard Mater. 2011;193:27–36.
Şahan T, Öztürk D. Investigation of Pb(II) adsorption onto pumice samples: application of optimization method based on fractional factorial design and response surface methodology. Clean Technol Environ Policy. 2014;16:819–31.
Rostamian R, Najafi M, Rafati AA. Synthesis and characterization of thiol-functionalized silica nano hollow sphere as a novel adsorbent for removal of poisonous heavy metal ions from water: kinetics, isotherms and error analysis. Chem Eng J. 2011;171:1004–11.
Rafatullah M, Sulaiman O, Hashim R, Ahmad A. Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater. 2010;177:70–80.
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q. Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater. 2012;211–212:317–31.
Bhatnagar A, Jain AK. A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J Colloid Interface Sci. 2005;281:49–55.
Bhattacharya AK, Naiya TK, Mandal SN, Das SK. Adsorption, kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chem Eng J. 2008;137:529–41.
Repo E, Warchoł JK, Bhatnagar A, Sillanpää M. Heavy metals adsorption by novel EDTA-modified chitosan–silica hybrid materials. Colloid Interface Sci. 2011;358:261–7.
Samiey B, Cheng C, Wu J. Organic–inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: a review. Materials. 2014;7:673–726.
Ok YS, Yang JE, Zhang Y, Kim S, Chung D. Heavy metal adsorption by a formulated zeolite-Portland cement mixture. J Hazard Mater. 2007;147:91–6.
Eisinas A, Baltakys K, Siauciunas R. Utilisation of gyrolite with impure Cd2 ions in cement stone. Adv Cem Res. 2013;25:69–79.
Mehrali M, Seyed Shirazi SF, Baradaran S, Mehrali M, Metselaar HSC, Kadri NAB, Osman NAA. Facile synthesis of calcium silicate hydrate using sodium dodecyl sulfate as a surfactant assisted by ultrasonic irradiation. Ultrason Sonochem. 2014;21:735–42.
Zhao J, Zhu Y, Wu J, Zheng J, Zhao X, Lu B, Chen F. Chitosan-coated mesoporous microspheres of calcium silicate hydrate: environmentally friendly synthesis and application as a highly efficient adsorbent for heavy metal ions. J Colloid Interface Sci. 2014;418:208–15.
Ahmaruzzaman M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv Colloid Interface Sci. 2011;166:36–59.
Bankauskaite A, Baltakys K, Eisinas A, Zadaviciute S. Study on adsorption of heavy metal ions in wastewater by synthetic layered inorganic adsorbents. Desalin Water Treat. 2014;26:9144.
Zhao Z, Wei J, Li F, Qu X, Shi L, Zhang H, Yu Q. Synthesis, characterization and hexavalent chromium adsorption characteristics of aluminum-and sucrose-incorporated tobermorite. Materials. 2017;10:597.
Okano K, Uemoto M, Kagami J, Miura K, Aketo T, Toda M, Honda K, Ohtake H. Novel technique for phosphorus recovery from aqueous solutions using amorphous calcium silicate hydrates (A-CSHs). Water Res. 2013;47:2251–9.
Plusquellec G, Nonat A. Interactions between calcium silicate hydrate (C–S–H) and calcium chloride, bromide and nitrate. Cem Concr Res. 2016;90:89–96.
Rajasekaran G. Sulphate attack and ettringite formation in the lime and cement stabilized marine clays. Ocean Eng. 2005;32:1133–59.
Cvetković VS, Purenović JM, Purenović MM, Jovićević JN. Interaction of Mg-enriched kaolinite–bentonite ceramics with arsenic aqueous solutions. Desalination. 2009;249:582–90.
Lu L, Xiang C, He Y, Wang F, Hu S. Early hydration of C3S in the presence of Cd2+, Pb2+ and Cr3+ and the immobilization of heavy metals in pastes. Constr Build Mater. 2017;152:923–32.
Niuniavaite D, Baltakys K, Dambrauskas T. The adsorption kinetic parameters of Co2 ions by α-C2SH. Buildings. 2018;8:10.
Dambrauskas T, Baltakys K, Eisinas A. Formation and thermal stability of calcium silicate hydrate substituted with Al3 ions in the mixtures with CaO/SiO2 = 15. J Therm Anal Calorim. 2018;131:501–12.
Baltakys K, Siauciunas R, Gendvilas R, Eisinas A, Dambrauskas T, Kitrys S. Physically and chemically bound H2O in the α-C2S hydrate structure. J Therm Anal Calorim. 2014;118:807–16.
Vargas AMM, Cazetta AL, Kunita MH, Silva TL, Almeida VC. Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): study of adsorption isotherms and kinetic models. Chem Eng J. 2011;168:722–30.
Wu B, Ye G. Carbonation mechanism of different kind of CSH: rate and products. Concr Suppl Cement Mater. 2016;113:163–272.
He Y, Zhao X, Lu L, Struble LJ, Hu S. Effect of C/S ratio on morphology and structure of hydrothermally synthesized calcium silicate hydrate. J Wuhan Univ Technol. 2011;26:770–3.
Yu P, Kirkpatrick RJ, Poe B, McMillan PF, Cong X. Structure of calcium silicate hydrate (C–S–H): near-, mid-, and far-infrared spectroscopy. J Am Ceram Soc. 1999;82:742–8.
Garbev K, Gasharova B, Beuchle G, Kreisz S, Stemmermann P. First observation of α-Ca2[SiO3(OH)](OH)–Ca6[Si2O7][SiO4](OH)2 phase transformation upon Thermal Treatment in Air. J Am Ceram Soc. 2008;91:263–71.
Crini G, Peindy HN, Gimbert F, Robert C. Removal of CI Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep Purif Technol. 2007;53:97–110.
Zhuang X, Wan Y, Feng C, Shen Y, Zhao D. Highly efficient adsorption of bulky dye molecules in wastewater on ordered mesoporous carbons. Chem Mater. 2009;21:706–16.
Southam DC, Lewis TW, McFarlane AJ, Johnston JH. Amorphous calcium silicate as a chemisorbent for phosphate. Curr Appl Phys. 2004;4:355–8.
Southam DC, Lewis TW, McFarlane AJ, Borrmann T, Johnston JH. Calcium–phosphorus interactions at a nano-structured silicate surface. J Colloid Interface Sci. 2008;319:489–97.
Diamond S. The microstructure of cement paste in concrete. Cem Concr Res. 1986;1:122–47.
Franus W, Panek R, Wdowin M. SEM investigation of microstructures in hydration products of Portland cement. In: 2nd international multidisciplinary microscopy and microanalysis congress; 2015, p. 105–12.
Becker O, Varley RJ, Simon GP. Thermal stability and water uptake of high performance epoxy layered silicate nanocomposites. Eur Polym J. 2004;40:187–95.
Gallucci E, Zhang X, Scrivener KL. Effect of temperature on the microstructure of calcium silicate hydrate (C–S–H). Cem Concr Res. 2013;53:185–95.
Liu X, Ding C. Plasma-sprayed wollastonite 2 M/ZrO2 composite coating. Surf Coat Technol. 2003;172:270–8.
Xue H, Wang G, Hu M, Chen B. Modification of wollastonite by acid treatment and alkali-induced redeposition for use as papermaking filler. Powder Technol. 2015;276:193–9.
Nour WMN, Mostafa AA, Ibrahim DM. Recycled wastes as precursor for synthesizing wollastonite. Ceram Int. 2008;34:101–5.
Acknowledgements
This work was funded by a Grant (No. S-MIP–17-92) from the Research Council of Lithuania.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niuniavaite, D., Baltakys, K., Dambrauskas, T. et al. Cu2+, Co2+ and Cr3+ adsorption by synthetic dibasic calcium silicate hydrates and their thermal stability in a 25–1000 °C temperature range. J Therm Anal Calorim 138, 2241–2249 (2019). https://doi.org/10.1007/s10973-019-08795-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08795-4