Abstract
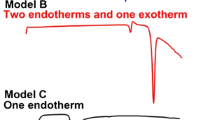
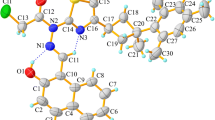
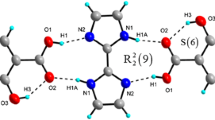
The pharmaceutical cocrystal of salicylic acid (C7H6O3 or H2Sal) and salicylamide (C7H7NO2 or SAM) was synthesized and characterized by various techniques. The differential scanning calorimetry results confirmed the eutectic fusion showing the characteristic of the endothermic sharp peak of the solidus temperature at 108 °C. X-ray crystal structure of cocrystal is orthorhombic with space group Pna2(1). Cocrystal consists of H2Sal and a distorted phenolic group of SAM. The packing diagram of cocrystal H2Sal·SAM clearly confirmed the \(R_{8}^{2}\) acid–amide dimer heterosynthons and other inter- and intramolecular interaction bonds to stabilize the structure. In addition, the strength of the hydrogen bonds is studied using the vibrational spectral measurements, confirming the band shifting due to the intermolecular interactions. The identity of compounds by matching the absorbance spectrum was confirmed by ultraviolet spectroscopy technique. Furthermore, the experimental studies were supported by calculation results using density functional B3LYP methods with the standard 6-311++G(d,p) basis set level. The parameters such as bond lengths, bond angles and Mulliken atomic charges values have been calculated and compared, confirmed the interactions and charge transfers. The frontier molecular orbitals (HOMO–LUMO) illustrated the lower band-gap value suggesting the possible pharmaceutical activity of this as obtained H2Sal·SAM cocrystal.










Similar content being viewed by others
References
Brittain HG. Cocrystal systems of pharmaceutical interest: 2010. Cryst Growth Des. 2012;12:1046–54.
Jones W, Samuel Motherwell WD, Trask AV. Pharmaceutical cocrystals: an emerging approach to physical property enhancement. MRS Bull. 2006;31:875–9.
Aitipamula S, Wong ABH, Chow PS, Tan RBH. Pharmaceutical cocrystals of ethenzamide: structural, solubility and dissolution studies. Cryst Eng Commun. 2012;14:8515–24.
Singh M, Rai RN, Rai US. Synthesis, crystal growth and physicochemical studies on a novel organic inter-molecular compound; 3,5-dinitrobenzoic acid and salicylamide system. J Cryst Growth. 2015;419:114–22.
Zhou Z, Chan HK, Sung HH-Y, Tong HHY, Zheng Y. Identification of new cocrystal systems with stoichiometric diversity of salicylic acid using thermal methods. Pharm Res. 2016;33:1030–9.
Manin AN, Voronin AP, Drozd KV, Manin NG, Bauer-Brandl A, Perlovich GL. Cocrystal screening of hydroxybenzamides with benzoic acid derivatives: a comparative study of thermal and solution-based methods. Eur J Med Sci. 2014;65:56–64.
Surov AO, Manin AN, Voronin AP, Churakov AV, Perlovich GL, Vener MV. Weak interactions cause packing polymorphism in pharmaceutical two-component crystals. The case study of the salicylamide cocrystal. Cryst Growth Des. 2017;17:1425–37.
Évora AOL, Castro RAE, Maria TMR, Ramos Silva M, ter Horst JH, Canotilho J, Eusébio MES. A thermodynamic based approach on the investigation of a diflunisal pharmaceutical co-crystal with improved intrinsic dissolution rate. Int J Pharm. 2014;466:68–75.
Elbagerma MA, Edwards HGM, Munshi T, Scowen IJ. Identification of a new co-crystal of salicylic acid and benzamide of pharmaceutical relevance. Anal Bioanal Chem. 2010;397:137–46.
Manin AN, Voronin AP, Manin NG, Vener MV, Shishkina AV, Lermontov AS, Perlovich GL. Salicylamide cocrystals: screening, crystal structure, sublimation thermodynamics, dissolution, and solid-state DFT calculations. J Phys Chem B. 2014;118:6803–14.
Seato CC, Parkin A. Making benzamide cocrystals with benzoic acids: the influence of chemical structure. Cryst Growth Des. 2011;11:1502–11.
Nordstrom FL, Rasmuson AC. Solubility and melting properties of salicylic acid. J Chem Eng Data. 2006;51:1668–71.
Nordstrom FL, Rasmuson AC. Solubility and melting properties of salicylamide. J Chem Eng Data. 2006;51:1775–7.
Sasada Y, Takano T, Kakudo M. Crystal structure of salicylamide. Bull Chem Soc Jpn. 1964;37(7):940–6.
Arjunan V, Kalaivani M, Ravindran P, Mohan S. Structural, vibrational and quantum chemical investigations on 5-chloro-2-hydroxybenzamide and 5-chloro-2-hydroxybenzoic acid. Spectrochim Acta Part A. 2011;79:1886–95.
Bartoszek-Adamska E, Dega-Szafran Z, Krociak M, Jaskolski M, Szafran M. Hydrogen bonds in 1:1 complex of piperidine-3-carboxylic acid with salicylic acid. J Mol Struct. 2009;920:68–74.
Boczar M, Boda L, Wojcik MJ. Theoretical modeling of infrared spectra of hydrogen-bonded crystals of salicylic acid. Spectrochim Acta A. 2006;64:757–60.
Munshi P, Guru Row TN. Intra- and intermolecular interactions in small bioactive molecules: cooperative features from experimental and theoretical charge-density analysis. Acta Cryst. 2006;B62:612–26.
Esrafili MD. Intra- and inter-molecular interactions in salicylic acid -theoretical calculations of 17O and 1H chemical shielding tensors and QTAIM analysis. Can J Chem. 2011;89:1410–8.
Kwon Y. Theoretical study on salicylic acid and its analogues: intramolecular hydrogen bonding. J Mol Struct (Theochem). 2000;532:227–37.
Karthick T, Balachandran V, Perumal S, Nataraj A. Spectroscopic studies, HOMO–LUMO and NBO calculations on monomer and dimer conformer of 5-nitrosalicylic acid. J Mol Struct. 2011;1005:192–201.
Velcheva EA, Stamboliyska BA. Structural changes caused by the conversion of 2-hydroxybenzamide salicylamide into the oxyanion. J Mol Struct. 2008;875:264–71.
Trask AV, Jones W. Crystal engineering of organic cocrystals by the solid-state grinding approach. Top Curr Chem. 2005;254:41–70.
Karabacak M, Sinha L, Prasad O, Cinar Z, Cinar M. The spectroscopic (FT-Raman, FT-IR, UV and NMR), molecular electrostatic, potential, polarizability and hyperpolarizability, NBO and HOMO–LUMO analysis of monomeric and dimeric structures of 4-chloro-3,5-dinitrobenzoic acid. Spectrochim Acta Part A. 2012;93:33–46.
Sheldrick GM. A short history of SHELX. Acta Crystallogr Sect A. 2008;64:112.
Brandenburg K. Diamond. Bonn: Cryst Impact GbR; 1999.
Frisch J, Trucks GW, et al. Gaussian 98. Pittsburgh(PA): Gaussian Inc; 1998.
Rai US, Singh M, Rai RN. Some physicochemical studies on organic eutectics and intermolecular compounds. J Therm Anal Calorim. 2017;130:967–74.
Fernandes RP, do Nascimento ALCS, Carvalho ACS, Teixeira JA, Ionashiro M, Caires JC. Mechanochemical synthesis, characterization, and thermal behavior of meloxicam cocrystals with salicylic acid, fumaric acid, and malic acid. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08118-7.
Landolt HH. Schmelzgleichgewichte, Vol. II, Part 3. 6th ed. Berlin: Springer; 1950. p. 1831–910.
Babhair SA, Al-Badr AA, Aboul-Enein HY. Salicylamide in analytical profiles of drug substances. In: Florey K, editor. Analytical profiles of drug substances, vol. 13. Orlando: Academic Press; 1984. p. 521–51.
Trivedi MK, Branton A, Trivedi D, Shettigar H, Bairwa K, Jana S. Fourier transform infrared and ultraviolet-visible spectroscopic characterization of biofield treated salicylic acid and sparfloxacin. Nat Prod Chem Res. 2015;3:1–6.
Amalanathan M, Rastogi VK, Hubert Joe I, Palafox MA, Tomar R. Density functional theory calculations and vibrational spectral analysis of 3,5-dinitrobenzoic acid. Spectrochim Acta Part A. 2011;78:1437–44.
Acknowledgements
We gratefully acknowledge the Thaksin University for the research grants via the Research and Development Institute, Thaksin University (RDITSU), and the National Research Management System (NRMS); project codes: 111006 and 184788.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phetmung, H., Musikapong, K. & Srichana, T. Thermal analysis, structure, spectroscopy and DFT calculations of a pharmaceutical cocrystal of salicylic acid and salicylamide. J Therm Anal Calorim 138, 1207–1220 (2019). https://doi.org/10.1007/s10973-019-08794-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08794-5