Abstract
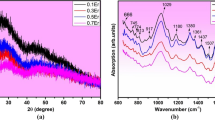
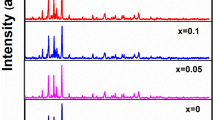
Zinc phosphate–germanate samples doped with Er3+ ions and co-doped with silver nanoparticles in the 59.7KPO3–0.3Ag(NPs)–20ZnO–(20 − x)GeO2–xEr2O3 system where x ≤ 5 mol% were prepared by melting–quenching technique. The mentioned system was studied by X-ray diffraction (XRD), differential thermal analysis (DTA) and ultraviolet–visible (UV–Vis) spectroscopy, too. XRD analysis shows the presence of amorphous state for low contents of erbium and the presence of the ErPO4 (tetragonal, body-centered lattice) crystalline phase beside the amorphous phase for high contents of erbium (x ≥ 3 mol%). DTA investigation permitted the identification of some thermal parameters such as glass transition temperature, crystallization temperature and melting temperature. From these data, other two important parameters were calculated: the fragility index and the activation energy of glass transition. In our case, the obtained data reveal a good thermal stability for the matrix of the studied system. The increase in the content of erbium ions leads to more fragile glasses and glass ceramic samples. The DTA results also show that the samples are obtained from KS liquids. The UV–Vis spectroscopy shows seven f–f electronic transitions for the studied system, due to the presence of erbium ions. From the obtained UV–Vis data, the bonding parameter that shows an ionic character of the bonds from the glass ceramic network was calculated. Also, the values of optical band gap energy (\(E_{\text{g}}^{\text{opt}}\)) show that when the erbium ions content increases, the \(E_{\text{g}}^{\text{opt}}\) values decrease due probably to the amount of non-bridging oxygen atoms from the network.




Similar content being viewed by others
References
Gomes JF, Lima AMO, Sandrini M, Medina AN, Steimacher A, Pedrochi F, Barboza MJ. Optical and spectroscopic study of erbium doped calcium borotellurite glasses. Opt Mater. 2017;66:211–9.
Umar SA, Halimah MK, Chan KT, Latif AA. Physical, structural and optical properties of erbium doped rice husk silicate borotellurite (Er-doped RHSBT) glasses. J Non-Cryst Solids. 2017;472:31–8.
Silarska K, Sroda M, Pasierb P. Application of DTA/DSC and dilatometry for optimization of Ba–Ce–Y–P–Si–O glass phase for composite protonic conductors based on BaCe0.9Y0.1O3−δ. J Therm Anal Calorim. 2018;133(1):87–93.
Gautam CR, Das S, Gautam SS, Madheshiya A, Singh AK. Processing and optical characterization of lead calcium titanate borosilicate glass doped with germanium. J Phys Chem Solids. 2018;115:180–6.
Tang Y, Shih K, Li M, Wu P. Zinc immobilization in simulated aluminum-rich waterworks sludge systems. Proc Environ Sci. 2016;31:691–7.
Qi Y, Zhou Y, Wu L, Yang F, Peng S, Zheng S, Yin D, Wang X. Annealing time dependent 1.53 µm fluorescence enhancement in Er3+-doped tellurite glasses containing silver NPs. Mater Lett. 2014;125:56–8.
Tauc J. Amorphous and liquid semiconductors. London: Plenum; 1974.
Davis EA, Mott NF. Conduction in non-crystalline systems conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos Mag. 1970;22:903–22.
Dietzel A. Glass structure and glass properties. Glasstech Berl. 1968;22:41–50.
Hruby A. Evaluation of glass-forming tendency by means of DTA. Physica B. 1972;22:1187–93.
Hruby A. Glass-forming tendency in the GeSx system. Physica B. 1973;23:1263–72.
Saad M, Poulin M. Glass forming ability criteria. Mater Sci Forum. 1987;19–20:11–8.
Rahvard MM, Tamizifar M, Boutorabi SMA. Non-isothermal crystallization kinetics and fragility of Zr56Co28Al16 and Zr56Co22Cu6Al16 bulk metallic glasses. J Therm Anal Calorim. 2018;134(2):903–14.
Kumar A, Ram IS, Kumar S, Ram J, Upadhyay AN, Singh K. Glass-forming ability and thermal stability of Se100−x(Ge2Sb2Te5)x glassy alloys. J Therm Anal Calorim. 2018;134(2):923–31.
Kissinger HE. Reaction kinetics in thermal analysis. Anal Chem. 1957;29:1702–6.
Saxena NS. Phase transformation kinetics and related thermodynamic and optical properties in chalcogenides glasses. J Non-Cryst Solids. 2004;161:345–6.
Assadi AA, Damak K, Lachheb R, Herrmann A, Yousef E, Russel C, Maâlej R. Spectroscopic and luminescence characteristics of erbium doped TNZL glass for lasing materials. J Alloys Compd. 2015;620:129–36.
Sinha SP. Complexes of the rare earths. Oxford: Pergamon Press; 1966.
Carnall WT, Fields PR, Rajnak K. Electronic Energy Levels in the Trivalent Lanthanide Aquo Ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J Chem Phys. 1968;49:4424–42.
Bolundut L, Culea E, Borodi G, Stefan R, Munteanu C, Pascuta P. Influence of Sm3+: Ag codoping on structural and spectroscopic properties of lead tellurite glass ceramics. Ceram Int. 2015;41:2931–9.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pop, L., Bolunduţ, L., Pascuta, P. et al. Influence of Er3+ ions addition on thermal and optical properties of phosphate–germanate system. J Therm Anal Calorim 138, 1895–1899 (2019). https://doi.org/10.1007/s10973-019-08145-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08145-4