Abstract
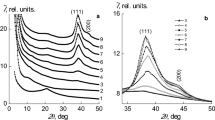
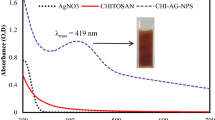
Thermal stability, structural peculiarities and thermal destruction of functional groups of the matrix, which are responsible for the stabilization of the nanosystems, of the gold- and silver-containing medicinally important water-soluble nanocomposites synthesized from therapeutic humic substances, isolated from brown coal of Baga Nuur field of Mongolian sources, have been studied. A crucial problem in biomedical and engineering application of the silver- and gold-containing nanocomposites is their stability upon heating, for example, during sterilization at high temperatures in medicine or laser action, which can heat a system, in plasmon technologies. All compounds have been investigated by complex modern physical–chemical methods (electron paramagnetic resonance and infrared spectroscopies, X-ray diffraction analysis, transmission electron microscopy and others). Thermal analysis of Ag0 and Au0 nanocomposites on the basis of humic matrices, as well as the initial humic substance, has been carried out. It is shown that the thermal decomposition of the nanocomposites depends on the nature of the metal. It is found that stable zero-valent nanoparticles of noble metals with an average particle size of 6–17 nm are formed in a natural matrix. The nanocomposites obtained are aggregatively stable for a long time, preserving their properties intact, that is extremely important for promising medicinal substances.





Similar content being viewed by others
References
Boisselier E, Didier A. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–82.
Hill JW. Collodial silver medical uses. Toxicology & manufacture. Washington: Clear Springs Press; 2009.
Daraee H, Eatemadi A, Abbasi E, Aval SF, Kouhi M, Akbarzadeh A. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:410–22.
Pomogailo AD, Dzhardimalieva GI. Nanostructured materials preparation via condensation ways. Dordrecht: Springer; 2014.
Pallem VL, Stretz HA, Wells MJM. Evaluating aggregation of gold nanoparticles and humic substances using fluorescence spectroscopy. Environ Sci Technol. 2009;43:7531–5.
Stevenson FJ. Humus chemistry: genesis, composition and reactions. 2nd ed. New York: Willey; 1994.
Sutton R, Sposito G. Molecular structure in soil humic substances: the new view. Environ Sci Technol. 2005;39:9009–15.
Klöcking R, Helbig B. Medical aspect and applications of humic substances. In: Steinbüchel A, Marchessault RH, editors. Biopolymers for medical and pharmaceutical applications. Weinheim: Wiley; 2005. p. 3–16.
Xu J, Wu J, He Y. Functions of natural organic matter in changing environment. Dordrecht: Springer; 2013.
Steinberg CEW. Ecology of humic substances in freshwaters. Berlin: Springer; 2003.
Perminova IV, Hatfield K, Hertkorn N. Use of humic substances to remediate polluted environments: from theory to practice. Dordrecht: Springer; 2005.
Piccolo A. The supramolecular structure of humic substances. Soil Sci. 2001;166:810–32.
Khutsishvili SS, Vakul’skaya TI, Aleksandrova GP, Sukhov BG. Strong stabilization properties of humic substance matrixes for silver bionanocomposites. Micro Nano Lett. 2017;12:418–21.
Antonelli ML, Calace N, Fortini C, Petronio BM, Pietroletti M, Pusceddu P. Calorimetric investigation of complex formation of some humic compounds. J Therm Anal Calorim. 2002;70:291–7.
Lesnichaya MV, Aleksandrova GP, Dolmaa G, Sapozhnikov AN, Sukhov BG, Regdel D, Trofimov BA. Synthesis of silver-containing nanocomposites based on humic substances of brown coal and their antioxidant activity. Dokl Chem. 2014;456:72–5.
Lesnichaya MV, Aleksandrova GP, Sukhov BG, Trofimov BA, Dolmaa G, Nomintsetseg B, Regdel D, Sapozhnikov AN. Features of gold nanoparticle formation in matrices of humic substances of different origin. Dokl Chem. 2015;460:13–6.
Kučerík J, Kislinger J, Majzlík P, Pekař M. Correlation of humic substances chemical properties and their thermo-oxidative degradation kinetics. J Therm Anal Calorim. 2009;98:207–14.
Rotaru A, Nicolaescu I, Rotaru P, Neaga C. Thermal characterization of humic acids and other components of raw coal. J Therm Anal Calorim. 2008;92:297–300.
Tikhonov NI, Khutsishvili SS, Lesnichaya MV, Dolmaa G, Vakul’skaya TI, Aleksandrova GP, Sukhov BG. Paramagnetic properties and antioxidant activity of metal-containing bionanocomposites based on humic substances. Magn Reson Solids. 2016;18:16104.
Poole CP. Electron spin resonance: a comprehensive treatise on experimental techniques. 2nd ed. Dover: Dover Publications; 1997.
Barret CA, Massalsky TB. structure of metals. New York: McGraw-Hill; 1966.
Pilawa B, Więckowski AB. Groups of paramagnetic centres in coal samples with different carbon contents. Res Chem Intermed. 2007;33:825–39.
González-Pérez M, Martin-Neto L, Colnago LA, Milori DMBP, de Camargo OA, Berton R, Bettiol W. Characterization of humic acids extracted from sewage sludge-amended oxisols by electron paramagnetic resonance. Soil Till Res. 2006;91:95–100.
Eichelbaum M, Rademann K, Hoell A, Tatchev DM, Weigel W, Stößer R, Pacchioni G. Photoluminescence of atomic gold and silver particles in soda-lime silicate glasses. Nanotechnology. 2008;19:135701.
Khutsishvili SS, Tikhonov NI, Lesnichaya MV, Dolmaa G, Vakul’skaya TI, Aleksandrova GP, Sukhov BG. Paramagnetic bioactive silver- and gold-containing nanocomposites based on humic substances. Funct Mater Lett. 2017;10:1650077.
Moon HR, Kim JH, Suh MP. Redox-active porous-organic framework producing silver nanoparticles from AgI ions at room temperature. Angew Chem Int Ed Engl. 2005;44:1261–5.
Lesnichaya MV, Sukhov BG, Aleksandrova GP, Gasilova ER, Vakul’skaya TI, Khutsishvili SS, Sapozhnikov AN, Klimenkov IV, Trofimov BA. Chiroplasmonic magnetic gold nanocomposites produced by one-step aqueous method using κ-carrageenan. Carbohydr Polym. 2017;175:18–26.
Mercê ALR, Landaluze JS, Mangrich AS, Szpoganicz B, Sierakowski MR. Complexes of arabinogalactan of Pereskia aculeata and Co2+, Cu2+, Mn2+, and Ni2+. Biores Technol. 2001;76:29–37.
Tarasova OA, Tatarinova IV, Vakul’skaya TI, Khutsishvili SS, Smirnov VI, Klyba LV, Prozorova GF, Mikhaleva AI, Trofimov BA. Propargyloxy- and allenyloxymethylferrocenes: synthesis and oligomerization. J Organomet Chem. 2013;745–746:1–7.
Kučerík J, Kovář J, Pekař M. Thermoanalytical investigation of lignite humic acids fractions. J Therm Anal Calorim. 2004;76:55–65.
Ur’yash VF, Larina VN, Kokurina NY, Novoselova NV. The thermochemical characteristics of cellulose and its mixtures with water. Russ J Phys Chem A. 2010;84:915–21.
Jakab E, Bora Á, Sebestyén Z, Borsa J. Thermal decomposition of chemically treated cellulosic fibres. J Therm Anal Calorim. 2018;132:433–43.
Provenzano MR, Senesi N. Thermal properties of standard reference humic substances by differential scanning calorimetry. J Therm Anal Calorim. 1999;57:517–26.
Chukhareva NV, Shishmina LV, Novikov AA. Kinetics of the degradation of peat humic acids. Solid Fuel Chem. 2003;37:30–41.
Martyniuk H, Więkowska J, Lipman J. The study of influence of metal ions on thermal decomposition of humic acids. J Therm Anal Calorim. 2001;65:711–21.
Krutyakov YA, Kudrinskiy AA, Olenin AY, Lisichkin GV. Synthesis and properties of silver nanoparticles: advances and prospects. Russ Chem Rev. 2008;77:233–57.
Ramezanzadeh B, Attar MM, Farzam M. Effect of ZnO nanoparticles on the thermal and mechanical properties of epoxy-based nanocomposite. J Therm Anal Calorim. 2011;103:731–9.
Babu VR, Kim C, Kim S, Ahn C, Lee Y. Development of semi-interpenetrating carbohydrate polymeric hydrogels embedded silver nanoparticles and its facile studies on E. coli. Carbohydr Polym. 2010;81:196–202.
Aleksandrova GP, Lesnichaya MV, Myachin YA, Sukhov BG, Trofimov BA. Effect of silver nanoparticles on the thermal characteristics of nanocomposites of galactose-containing polysaccharides. Dokl Chem. 2011;439:187–9.
Gendler TS, Novakova AA, Prudnikov VN. Comparative analysis of γ-Fe2O3 nanoparticles magnetic interactions in different polymeric nanocomposites. Solid State Phenom. 2009;152:269–72.
Zakis GF, transl. ed. Joyce T, Brezny R. Functional analysis of lignins and their derivatives. Tappi Pr, New York; 1994.
Lishtvan II, Yanuta YG, Abramets AM, Monich GS, Glukhova NS, Aleinikova VN. Interactions of humic acids with metal ions in the water medium. J Water Chem Technol. 2012;34:211–7.
Acknowledgements
The authors are grateful to the Baikal Analytical Center for the special measurements. Thermoanalytical studies are performed by Penzik M.V. and Kozlov A.N. in the framework of the scientific project III.17.1.2 of the program of fundamental research of SB RAS, reg. No AAAA-A17-117030310448-0 on the equipment of the “High-temperature circuit” (ESI SB RAS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khutsishvili, S.S., Tikhonov, N.I., Pavlov, D.V. et al. Gold- and silver-containing bionanocomposites based on humic substances extracted from coals. J Therm Anal Calorim 137, 1181–1188 (2019). https://doi.org/10.1007/s10973-019-08059-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08059-1