Abstract
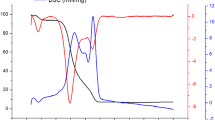
In order to investigate the influence of dissociation reaction on thermal decomposition of ammonium nitrate (AN), biochar was selected as an adsorbent to interfere with the dissociation of AN. The TG-DSC results showed that the notable exothermic reaction of AN with the presence of 2% or 7% biochar took place. The decomposition temperature of AN decreased with increasing amount of biochar. The notable knee point was found in the TG curves. The activation energy of AN with biochar in the initial stage was higher than that of AN itself. Remote sensing Fourier transform infrared experiments found biochar induced AN decomposition at about 190 °C, which was also confirmed by the TG-MS results. After dissociation reaction, HNO3 (g) and NH3 (g) were adsorbed and crystalline of AN was formed on the surface of biochar. With the increasing temperature, NH3 escaped from the surface of biochar, while HNO3 (g) was stayed in biochar. HNO3 (g) catalyzed the thermal decomposition of AN and also reacted with biochar. The results indicated that dissociation reaction of AN played an important role during AN thermal decomposition process. When dissociation reaction was changed, the thermal decomposition reaction of AN would also change, catalysis or inhibition AN thermal decomposition. It is a useful reference to guide the AN additives selection and to understand the mechanism for the AN decomposition accident.















Similar content being viewed by others
References
Yang M, Chen X, Wang Y, et al. Comparative evaluation of thermal decomposition behavior and thermal stability of powdered ammonium nitrate under different atmosphere conditions. J Hazard Mater. 2017;337:10–9.
Jos J, Mathew S. Ammonium nitrate as an eco-friendly oxidizer for composite solid propellants: promises and challenges. Crit Rev Solid State Mater Sci. 2016;42:1–29.
Oommen C, Jain SR. Ammonium nitrate: a promising rocket propellant oxidizer. J Hazard Mater. 1999;67:253–81.
Shalini C, Pragnesh ND. Review on thermal decomposition of ammonium nitrate. J Energ Mater. 2013;31:1–26.
Sinditskii V, Egorshev V, Levshenkov A, et al. Ammonium nitrate: combustion mechanism and the role of additives. Propell Explos Pyrotech. 2005;30:269–80.
Pittman W, Han Z, Harding B, et al. Lessons to be learned from an analysis of ammonium nitrate disasters in the last 100 years. J Hazard Mater. 2014;280:472–7.
Tomoki N, Makoto K. Burning characteristics of ammonium nitrate-based composite propellants supplemented with MnO2. Propell Explos Pyrotech. 2013;38:87–94.
Makoto K, Tomoki N. Thermal decomposition behaviors and burning characteristics of AN/RDX-based composite propellants supplemented with MnO2 and Fe2O3. J Energ Mater. 2015;33:288–304.
Tomoki N, Makoto K. Burning characteristics of ammonium nitrate-based composite propellants supplemented with Fe2O3. Propell Explos Pyrotech. 2013;38:547–54.
Vargeese AA, Muralidharan K. Anatase–brookite mixed phase nano TiO2 catalyzed homolytic decomposition of ammonium nitrate. J Hazard Mater. 2011;192:1314–20.
Vargeese AA, Muralidharan K, Krishnamurthy VN. Kinetics of nano titanium dioxide catalyzed thermal decomposition of ammonium nitrate and ammonium nitrate-based composite solid propellant. Propell Explos Pyrotech. 2015;40:260–6.
Kajiyama K, Izato Y, Miyake A. Thermal characteristics of ammonium nitrate, carbon, and copper (II) oxide mixtures. J Therm Anal Calorim. 2013;11:1475–80.
Tomoki N, Makoto K. Influence of Fe2O3 size on burning characteristics of ammonium nitrate/Fe2O3 propellants. J Propul Power. 2014;30:864–6.
Izato Y, Miyake A. Combustion characteristics of ammonium nitrate and carbon mixtures based on a thermal decomposition mechanism. Propell Explos Pyrotech. 2013;38:129–35.
Xu ZX, Xu GS, Fu XQ, et al. Thermal decomposition characteristics of ammonium nitrate (V) in the presence of Mn2O3/graphene oxides. Cent Eur J Energ Mater. 2017;14:636–59.
Xu ZX, Xu GS, Fu XQ, et al. The mechanism of nano-CuO and CuFe2O4 catalyzed thermal decomposition of ammonium nitrate. Nanomater Nanotechnol. 2016;6:1–10.
Sun J, Sun Z, Wang Q, et al. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate. J Hazard Mater. 2005;127:204–10.
Oxley JC, Smith JL, Rogers E, et al. Ammonium nitrate: thermal stability and explosivity modifiers. Thermochim Acta. 2002;384:23–45.
Li XR, Koseki H. Study on the contamination of chlorides in ammonium nitrate. Process Saf Environ Protect. 2005;83:31–7.
Wang S, Xu ZX, Wang Q. Thermal decomposition mechanism of emulsion explosives with phosphatide. J Therm Anal Calorim. 2016;124:1053–62.
Xu ZX, Liu DB, Hu YT, et al. Influence of iron ion on thermal behavior of ammonium nitrate and emulsion explosives. Cent Eur J Energ Mater. 2010;7:77–93.
Sudhakar AOR, Mathew S. Thermal behaviour of CuO doped phase-stabilised ammonium nitrate. Thermochim Acta. 2006;451:5–9.
Han Z, Sachdeva S, Papadaki MI, et al. Ammonium nitrate thermal decomposition with additives. J Loss Prevent Proc Ind. 2015;35:307–15.
Han Z, Sachdeva S, Papadaki MI, et al. Effects of inhibitor and promoter mixtures on ammonium nitrate fertilizer explosion hazards. Thermochim Acta. 2016;624:69–75.
Cagnina S, Rotureau P, Singh S, et al. Theoretical and experimental study of the reaction between ammonium nitrate and sodium salts. Ind Eng Chem Res. 2016;55:12183–90.
Xu L, Cheng JH, Liu P, et al. Production of bio-fuel oil from pyrolysis of plant acidified oil. Renew Energ. 2019;130:910–9.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Bekiaris G, Peltre C, Jensen LS, et al. Using FTIR-photoacoustic spectroscopy for phosphorus speciation analysis of biochars. Spectrochim Acta A. 2016;168:29–36.
Chen Q, Zheng J, Zheng L, et al. Classical theory and electron-scale view of exceptional Cd (II) adsorption onto mesoporous cellulose biochar via experimental analysis coupled with DFT calculations. Chem Eng J. 2018;350:1000–9.
Yuan JH, Xu RK, Zhang H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol. 2011;102:3488–97.
Xu ZX, Liu P, Xu GS, et al. Bio-fuel oil characteristic from catalytic cracking of hydrogenated palm oil. Energy. 2017;133:666–75.
Titirici MM, White RJ, Falco C, et al. Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energ Environ Sci. 2012;5(5):6796–822.
Singh B, Fang Y, Cowie BCC, et al. NEXAFS and XPS characterisation of carbon functional groups of fresh and aged biochars. Org Geochem. 2014;77:1–10.
Mukherjee A, Zimmerman AR, Harris W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma. 2011;163(3–4):247–55.
Usowicz B, Lipiec J, Łukowski M, et al. The effect of biochar application on thermal properties and albedo of loess soil under grassland and fallow. Soil Till Res. 2016;164:45–51.
Xu ZX, Wang Q, Fu XQ. Thermal stability and mechanism of decomposition of emulsion explosives in the presence of pyrite. J Hazard Mater. 2015;300:702–10.
Xu ZX, Wang Q, Zhu X, et al. Thermal stability of ammonium nitrate in high-temperature coal seam. J Therm Anal Calorim. 2017;130:1171–9.
Wu H, Han MC, Chan ChK. FTIR Characterization of polymorphic transformation of ammonium nitrate. Aerosol Sci Technol. 2007;41:581–8.
Thboret A, Sandorey C. Infrared spectra and crystalline phase transition of ammonium nitrate. Can J Chem. 1964;42:57–62.
Xu ZX, Wang Q, Fu XQ. Phase stability of ammonium nitrate with organic potassium salts. Cent Eur J Energ Mater. 2016;13:736–54.
da Cunha MCPM, Weber M, Nart FC. On the adsorption and reduction of NO3 − ions at Au and Pt electrodes studied by in situ FTIR spectroscopy. J Electroanal Chem. 1996;414:163–70.
Östmark H, Wallin S, Ang HG. Vapor pressure of explosives: a critical review. Propell Explos Pyrot. 2012;37:12–23.
Kajiyama K, Izato Y, Miyake A. Thermal characteristics of ammonium nitrate, carbon, and copper (II) oxide mixtures. J Therm Anal Calorim. 2013;113:1475–80.
Acknowledgements
This paper was supported by the Foundation of Jiangsu University of Advanced scholars (15JDG159) and the Natural Science Foundation of Jiangsu Colleges and Universities (16KJB150010).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, ZX., Cheng, JH., Wang, Q. et al. The influence of dissociation reaction on ammonium nitrate thermal decomposition reaction. J Therm Anal Calorim 136, 1415–1424 (2019). https://doi.org/10.1007/s10973-018-7808-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7808-4