Abstract
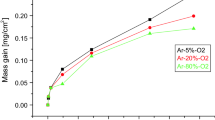
The oxidation behavior of U-6 mass% Zr alloy was studied by using thermogravimetric technique. Oxidation reaction was carried out by heating alloy sample in a stream of oxygen. Both isothermal and non-isothermal methods were used to study the kinetics of oxidation reaction. Model-free isoconversional method was used to derive the kinetic parameters. A single-step oxidation reaction was observed. The completion of oxidation reaction was ascertained by constancy of sample mass with respect to time and temperature. It was observed that under the favorable conditions of temperature, time and concentration of oxygen (Vpm of O2) alloy sample underwent ignition also. Hence, dependence of inception of ignition reaction on concentration of oxygen (Vpm of O2) was also studied. The effective activation energy obtained using isoconversional method for U-6 mass% Zr alloy was found to be 85 ± 7 kJ mol−1 for ‘α’ = 0.1 to 0.8 (non-isothermal experiments). It was inferred that prolonged exposure of the alloy to oxygen at room temperature resulted in its surface oxidation. The products of oxidation reaction did not result in a protective coating on the specimen.











Similar content being viewed by others
References
Cathcart JV, Pawel RE, Petersen GF. High temperature oxidation of uranium alloys. Oak Ridge National Laboratory: Oak Ridge; 1974.
Barnartt S, Charles RG, Gulbransen EA. Oxidation of 50 weight per cent uranium-zirconium alloy. J Electrochem Soc. 1957;104(4):218–21.
Matsui T, Yamada T, Ikai Y, Naito K. Oxidation of U-20 at.% Zr alloy in air at 423–1063 K. J Nucl Mater. 1993;199:143–8.
Matsui T, Yamada T, Ikai Y. Oxidation of U-10 at.% Zr alloy in air at 423–1028 K. J Nucl Mater. 1994;210:172–7.
Rao GAR, Venugopal V, Sood DD. Oxidation studies on U–Zr alloys. J Nucl Mater. 1994;209:161–8.
Schnizlein JG, Baker L, Bingle JR, Bingle JD. The ignition of binary alloys of uranium. J Nucl Mater. 1966;20:39–47.
Vyazovkin S, Charles AW. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Chetty KV, Radhakrishna J, Sayi YS, Balachander N, Venkataramana P, Natarajan PR. Radiochem Radioanal Lett. 1983;58:161–2.
Kaity S, Banerjee J, Nair MR, Ravi K, Dash S, Kutty TRG, Kumar A, Singh RP. Microstructural and thermophysical properties of U-6 mass% Zr alloy for fast reactor application. J Nucl Mater. 2012;427:1–11.
Kutty TRG, Kaity S, Kumar A. Impression creep behaviour of U-6% Zr alloy: role of Microstructure. Procedia Eng. 2013;55:561–5.
Brown M E, Dollimore D, Galwey A K. Comprehensive chemical kinetics. 22nd vol. 22. Amsterdam: Elsevier; 1988.
Ortega A. A simple and precise linear integral method for isoconversional data. Thermochim Acta. 2008;474:81–6.
Simon P. Isoconversional methods: fundamentals, meaning and application. J Therm Anal Calorim. 2004;76:123–32.
Jain A, Anthonysamy S. Oxidation of boron carbide powder. J Therm Anal Calorim. 2015;122:645–52.
Vyazovkin S. An approach to the solution of the inverse kinetic problem in the case of complex process. Part 4. Chemical reaction complicated by diffusion. Thermochim Acta. 1993;223:201–6.
Roberts AF. A review of kinetics data for the pyrolysis of wood and related substances. Combust Flame. 1970;14:261–72.
Williams FA. Combustion theory, Benjamin Cummings. Ohio: Menlo Park; 1985.
Nawada HP, Srirama Murti P, Seenivasan G, Anthonysamy S. Thermochim Acta. 1989;144:357–61.
Acknowledgements
The authors wish to thank Dr. B. P. Reddy, Head, Pyrochemical & Materials Processing Division for providing U-6 mass% Zr alloy sample for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, A., Sharma, B.K. & Manivannan, A. Oxidation behavior of U-6 mass% Zr alloy. J Therm Anal Calorim 136, 1285–1293 (2019). https://doi.org/10.1007/s10973-018-7779-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7779-5