Abstract
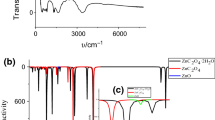
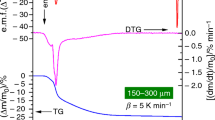
This study describes the physico-geometrical mechanism and overall kinetics for the multistep thermal dehydration of barium titanyl oxalate tetrahydrate (BTO). The thermal dehydration kinetics of BTO was studied at four different linear heating rates under non-isothermal conditions. The reaction kinetics was performed using differential scanning calorimetry (DSC) and the curves obtained were analysed using different isoconversional model-free equations and the values are found to be compatible with each other. The kinetic deconvolution principle is used for identifying the partially overlapped kinetic processes of the thermal dehydration of BTO, and it occurs in two stages. The overall reaction kinetics parameters calculated via kinetic deconvolution of the sample indicate the multistep nature of the process and the kinetic analysis of the non-isothermal data of this reaction model shows that the reaction is best described by Sestak–Berggren (m, n) empirical kinetic model. The prepared sample was identified and characterized by means of FT-IR, XRD, SEM, and TEM.













Similar content being viewed by others
References
Min C, Kim S, Lee C. Morphology of barium titanate particles produced by homogeneous precipitation. Bull Korean Chem Soc. 1997;18:600–3.
Pfaff G. Sol-gel synthesis of barium titanate powders of various compositions. J Mater Chem. 1992;2:591–4.
Hoffmann T, Doll T, Fuenzalida VM. Fabrication of BaTiO3 microstructures by hydrothermal growth. J Electrochem Soc. 1997;144:292–3.
Pechini MP. Barium titanium citrate, barium titanium and processes for producing same. Patent US 3231328. 1996.
Kim JG, Ha JG, Lim TW, Park K. Preparation of porous BaTiO3-based ceramics by high-energy ball-milling process. Mater Lett. 2006;60:1505–8.
Vinothini V, Singh P, Balasubramanian M. Synthesis of barium titanate nanopowder using polymeric precursor method. Ceram Int. 2006;32:99–103.
Ghosh S, Dasgupta S, Sen A, Maiti HS. Synthesis of barium titanate nanopowder by a soft chemical process. Mater Lett. 2007;61:538–41.
Jung YJ, Lim DY, Nho JS, Cho SB, Riman RE, Lee BW. Glycothermal synthesis and characterization of tetragonal barium titanate. J Cryst Growth. 2005;274:638–52.
Ragulya AV, Vasylkiv OO, Skorokhod VV. Synthesis and sintering of nanocrystalline barium titanate powder under nonisothermal conditions. I. Control of dispersity of barium titanate during its synthesis from barium titanyl oxalate. Powder Metall Met Ceram. 1997;36:170–5.
Bera J, Sarkar D. Formation of BaTiO3 from barium oxalate and TiO2. J Electroceram. 2003;11:131–7.
Wada S, Narahara M, Hoshina T, Kakemoto H, Tsurumi T. Preparation of nm-sized BaTiO3 particles using a new 2-step thermal decomposition of barium titanyl oxalate. J Mater Sci. 2003;38:2655–60.
Huang C, Chen K, Chiu P, Sze P, Wang Y. The novel formation of barium titanate nanodendrites. J Nanomed. 2014;2014:1–7.
Clabaugh WS, Swiggard EM, Gilchrist R. Preparation of barium titanyl oxalate tetrahydrate for conversion to barium titanate of high purity. J Res Nat Bur Stand (U.S.). 1956;56:289–91.
Kudaka K, Ilzumi K, Sasaki K. Preparation of stoichiometric barium titanyl oxalate tetrahydrate. J Am Ceram Soc Bull. 1982;61:1236.
Fang TT, Lin HB. Factors affecting the preparation of barium titanyl oxalate tetrahydrate. J Am Ceram Soc. 1989;72:1899–906.
Fang TT, Lin HB, Hwang JB. Thermal analysis of precursors of barium titanate prepared by co-precipitation. J Am Ceram Soc. 1990;73:3363–7.
Yamamura H, Watanabe A, Shirasaki S, Moriyoshi Y, Tanada M. Preparation of barium titanate by oxalate method in ethanol solution. Ceram Int. 1985;11:17–22.
Amala Sekar M, Dhanaraj G, Bhat HL, Patil KC. Synthesis of fine-particle titanates by the pyrolysis of oxalate precursors. J Mater Sci. 1992;3:237–9.
Otta S, Bhattamisra SD. Kinetics and mechanism of the thermal decomposition of barium titanyl oxalate. J Therm Anal. 1994;41:419–33.
Gallagher PK, Schrey F. Preparation of semiconducting titanates by chemical methods. J Am Ceram Soc. 1963;46:567.
Balek V, Kaisersberger E. Preparation of BaTiO3 by thermal decomposition of BTO simultaneously investigated by emanation thermal analysis, TG-DTA and EGA. Thermochim Acta. 1985;85:207–10.
Kiss K, Magder J, Vukasovigh MS, Lockhart RJ. Ferroelectrics of ultrafine particles size: 1. Synthesis of titanate powders of ultrafine particle size. J Am Ceram Soc. 1966;49:291.
Saburi O. Semiconducting bodies in the family of barium titanates. J Am Ceram Soc. 1961;44:54–63.
Gallagher PK, Thomson J. Thermal analysis of some barium and strontium titanyl oxalates. J Am Ceram Soc. 1965;48:644–7.
Swilam MN, Gadalla AM. Decomposition of barium titanyl oxalate and assesment of barium titanate produced at various temperatures. Trans J Brit Ceram Soc. 1975;74:159.
Yen FS, Chang CT, Chang YH. Characterization of BTO tetrahydrate. J Am Ceram Soc. 1990;73:3422.
Gopalakrishnamurthy HS, Rao MS, Kutty TRN. Thermal decomposition of titanyl oxalates–1. Barium titanyl oxalate. J Inorg Nucl Chem. 1975;37:891–8.
Sarada K, Muraleedharan K. Effect of addition of silver on the thermal decomposition kinetics of copper oxalate. J Therm Anal Calorim. 2016;123:643–51.
Fu XL, Fan XZ, Wang BZ, Huo H, Li JZ, Hu RZ. Thermal behavior, decomposition mechanism and thermal safety of 5, 7-diamino-4,6-dinitrobenzenfuroxan (CL-14). J Therm Anal Calorim. 2016;124:993–1001.
Atakol M, Atakol A, Yiğiter AÖ, Svoboda I, Atakol O. Investigation of energetic materials prepared by reactions of diamines with picryl chloride: synthesis, structure and thermal behaviour. J Therm Anal Calorim. 2017;127:1931–40.
Dollimore D, Griffiths DL, Nichoison D. The thermal decomposition of oxalates. Part II. Thermogravimetric analysis of various oxalates in air and in nitrogen. J Chem Soc. 1963;3:2617–23.
Koga N, Suzuki Y, Tatsuoka T. Thermal dehydration of magnesium acetate tetra hydrate: formation and in situ crystallization of anhydrous glass. J Phys Chem B. 2012;116:14477–86.
Kotru PN, Raina KK, Koul ML. The kinetics of solid-state decomposition of neodymium tartrate. Indian J Pure Appl Phys. 1987;25:220.
Schaube F, Koch L, Worner A, Steinha HM. A thermodynamic and kinetic study of the de- and rehydration of Ca(OH)2 at high H2O partial pressures for thermo-chemical heat storage. Thermochim Acta. 2012;538:9–20.
Fatu D. Kinetics of gypsum dehydration. J Therm Anal Calorim. 2001;65:213–20.
Modestov N, Poplankhin PV, Lyakhov NZ. Dehydration kinetics of lithium sulfate monohydrate single crystals. J Therm Anal Calorim. 2001;65:121–30.
Halawy SA, Fouad NE, Mohamed MA, Zaki MI. Kinetic and thermodynamic parameters of the decomposition of chromium chromate in different gas atmospheres. J Therm Anal Calorim. 2001;65:167–76.
Dalal PV, Saraf KB, Shimpi NG, Shah NR. Pyro and kinetic studies of barium oxalate crystals grown in agar gel. J Cryst Process Technol. 2012;2:156–60.
Horowitz HH, Metzger G. New analysis of thermogravimetric traces, analytical chemistry. Anal Chem. 1963;35:1464–8.
Freeman ES, Carroll B. The application of thermoanalytical decomposition of calcium oxalate mono-hydrate. J Phys Chem. 1958;62:394–7.
Nakamoto K. Infrared spectra of inorganic and co-ordination compounds. 2nd ed. NewYork: Wiley; 1969. p. 245.
Xiao CJ, Jin CQ, Wang XH. Crystal structure of dense nanocrystalline BaTiO3 ceramics. Mater Chem Phys. 2008;111:2–3.
Koga N, Sesták J, Simon P. Some fundamental and historical aspects of phenomenological kinetics in the solid state studied by thermal analysis. In: Sesták J, Simon P, editors. Thermal analysis of micro, nano- and non-crystalline materials. Berlin: Springer; 2013. p. 1–28.
Koga N, Sesták J, Simon P. Ozawa’s kinetic method for analyzing thermoanalytical curves. J Therm Anal Calorim. 2013;113:1527–41.
Bosewell PG. On the calculation of activation energies using a modified Kissinger method. J Therm Anal. 1980;18:353–8.
Tang W, Liu Y, Zhang H, Wang C. New approximate formula for Arrhenius temperature integral. Thermochim Acta. 2003;408:39–43.
Starink MJ. Activation energy determination for linear heating experiments: deviations due to neglecting the low temperature end of the temperature integral. J Mater Sci. 2007;42:483–9.
Wada T, Koga N. Kinetics and mechanism of the thermal decomposition of sodium per carbonate: role of the surface product layer. J Phys Chem A. 2013;117:1880–9.
Wada T, Nakano M, Koga N. Multistep kinetic behaviour of the thermal decomposition of granular sodium per carbonate: hindrance effect of the outer surface layer. J Phys Chem A. 2015;119:9749–60.
Yoshikawa M, Yamada S, Koga N. Phenomenological interpretation of the multistep thermal decomposition of silver carbonate to form silver metal. J Phys Chem C. 2014;118:8059–70.
Kitabayashi S, Koga N. Physico-geometrical mechanism and overall kinetics of thermally induced oxidative decomposition of tin(II) oxalate in air: formation process of micro structural tin(IV) oxide. J Phys Chem C. 2014;118:17847–61.
Koga N, Goshi Y, Yamada S, Perez-Maqueda LA. Kinetic approach to partially overlapped thermal decomposition processes; co-precipitated zinc carbonates. J Therm Anal Calorim. 2013;111:1463–74.
Koga N, Kasahara D, Kimura T. Aragonite crystal growth and solid-state aragonite-calcite transformation: a physico-geometrical relationship via thermal dehydration of included water. Cryst Growth Des. 2013;13:2238–46.
Koga N, Yamada S, Kimura T. Thermal decomposition of silver carbonate: phenomenology and physico-geometrical kinetics. J Phys Chem C. 2013;117:326–36.
Sanchez-Jimenez PE, Perejon A, Criado JM, Dianez MJ, Perez-Maqueda LA. Kinetic model for thermal dehydrochlorination of poly(vinyl chloride). Polymer. 2010;51:3998–4007.
Noda Y, Koga N. Phenomenological kinetics of the carbonation reaction of lithium hydroxide monohydrate: role of surface product layer and possible existence of a liquid phase. J Phys Chem C. 2014;118:5424–36.
Nusrath K, Muraleedharan K. Effect of nano-transition metal oxides of Fe, Co and Ni and ferrites of Co and Ni on the multistage thermal decomposition of oxalates of Ce(III). J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7648-2.
Sestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Cai J, Liu R. Weibull mixture model for modelling nonisothermal kinetics of thermally stimulated solid-state reactions: application to simulated and real kinetic conversion data. J Phys Chem B. 2007;111:10681–6.
Avrami M. Kinetics of phase change. III. Granulation, phase change, and microstructure. J Chem Phys. 1941;9:177–84.
Barmak K. A commentary on: Reaction kinetics in processes of nucleation and growth. Met Mater Trans A. 2010;41:2711–75.
Ferriol M, Gentilhomme A, Cochez M, Oget N, Mieloszynski JL. Thermal degradation of poly(methyl methacrylate) (PMMA): modelling of DTG and TG curves. Polym Degrad Stab. 2003;79:271–81.
Font R, Conesa JA, Molto J, Munoz M. Kinetics of pyrolysis and combustion of pine needles and cones. J Anal Appl Pyrol. 2009;85:276–86.
Lopez G, Aguado R, Olazar M, Arabiourrutia M, Bilbao J. Kinetics of scrap tyre pyrolysis under vacuum conditions. Waste Manag. 2009;29:2649–55.
Sanchez-Jimenez PE, Perejon A, Criado JM, Dianez MJ, Perez-Maqueda LA. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B. 2011;115:1780–91.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sindhu, N.V., Muraleedharan, K. Kinetic study of the multistep thermal behaviour of barium titanyl oxalate prepared via chemical precipitation method. J Therm Anal Calorim 136, 1295–1306 (2019). https://doi.org/10.1007/s10973-018-7777-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7777-7