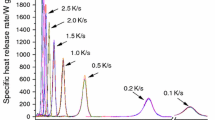
Abstract
Flammability studies are conducted to evaluate the behavior of materials exposed to fire. In this study, microscale combustion calorimetry (MCC) and cone calorimetry methods were applied to acquire the flammability characteristics of red and grey extruded polystyrene (XPS) samples. To understand the effect of changes between parameters, Pearson’s correlation coefficient was used to examine their linear relationships. From the research, moderate and weak correlations were recorded between the total heat release rates from both methods for red and grey XPS, respectively. Plotting peak heat release rate against heat release temperature for MCC and ignition temperature for cone test showed that 25, 35 and 50 kW m−2 incident heat fluxes of the cone test fall within 0.2 K s−1 and 0.5 K s−1 heating rates of MCC. Also, all the MCC parameters except char yield and total heat release presented good correlations with the cone calorimetry flammability characteristics. Hence, MCC could be used in conjunction with cone calorimetry to accurately and reliably assess the flammability of materials.









Similar content being viewed by others
Abbreviations
- \(\beta\) :
-
Heating rate in MCC test/K s−1
- \({\text{Bi}}\) :
-
Biot’s number
- \(c\) :
-
Specific heat/J g−1 K−1
- \(h\) :
-
Heat transfer coefficient/W m−2 K−1
- \(h_{\text{g}}\) :
-
Heat of gasification/MJ kg−1
- \(k\) :
-
Thermal conductivity/W m−1 K−1
- \(L_{\text{c}}\) :
-
Characteristic length/m
- \(m_{\text{o}}\) :
-
Initial mass of sample/g
- \(m_{\text{p}}\) :
-
Mass of residue/g
- \(\eta_{\text{c}}\) :
-
Heat release capacity/J g−1 K−1
- pHRR:
-
Peak heat release rate/W g−1
- pTemp:
-
Temperature at pHRR/°C
- \(q_{\text{in}}\) :
-
Incident heat flux/kW m−2
- \(q_{\hbox{max} }\) :
-
Maximum value of heat release rate per unit area/kW m−2
- THR:
-
Total heat release/kJ g−1
- T ig :
-
Ignition temperature/°C
- t ig :
-
Time to ignition/s
- \(\Delta T_{\text{ig}}\) :
-
Change in ignition temperature/°C
- T py :
-
Pyrolysis temperature/°C
- \(\rho\) :
-
Density/kg m−3
References
Lyon RE, Walters R. A microscale combustion calorimeter. Washington: Federal Aviation Administration; 2002.
Standard test method for determining flammability characteristics of plastics and other solid materials using microscale combustion calorimetry. ASTM D7309-13. 2013.
Walters RN, Lyon RE. Microscale combustion calorimeter for determining flammability parameters of materials. Evol Technol Compet Edge. 1997;42:1335–44.
Hostikka S, Matala A. Pyrolysis Model for Predicting the Heat Release Rate of Birch Wood. Combust Sci Technol. 2017;189(8):1373–93.
Lyon RE, Walters RN, Stoliarov SI. Screening flame retardants for plastics using microscale combustion calorimetry. Polym Eng Sci. 2007;47(10):1501–10.
Schartel B, Pawlowski KH, Lyon RE. Pyrolysis combustion flow calorimeter: a tool to assess flame retarded PC/ABS materials. Thermochim Acta. 2007;462(1–2):1–4.
Yang CQ, He Q, Lyon RE, Hu Y. Investigation of the flammability of different textile fabrics using micro-scale combustion calorimetry. Polym Degrad Stab. 2010;95(2):108–15.
Babrauskas V. The cone calorimeter. In: Hrtkey MJ, et al., editors. SFPE handbook of fire protection engineering. New York: Springer; 2016. p. 952–80.
ISO 5660-1:2002 Reaction-to-fire tests-heat release, smoke production and mass loss rate-part 1: heat release rate (cone calorimeter method).
Schartel B, Hull TR. Development of fire-retarded materials—interpretation of cone calorimeter data. Fire Mater. 2007;31(5):327–54.
Qin H, Zhang S, Zhao C, Feng M, Yang M, Shu Z, Yang S. Thermal stability and flammability of polypropylene/montmorillonite composites. Polym Degrad Stab. 2004;85(2):807–13.
Qin H, Su Q, Zhang S, Zhao B, Yang M. Thermal stability and flammability of polyamide 66/montmorillonite nanocomposites. Polymer. 2003;44(24):7533–8.
Zhao C, Qin H, Gong F, Feng M, Zhang S, Yang M. Mechanical, thermal and flammability properties of polyethylene/clay nanocomposites. Polym Degrad Stab. 2005;87(1):183–9.
Delichatsios M, Paroz B, Bhargava A. Flammability properties for charring materials. Fire Saf J. 2003;38(3):219–28.
Heskestad AW, Hovde PJ. Empirical prediction of smoke production in the ISO room corner fire test by use of ISO cone calorimeter fire test data. Fire Mater. 1999;23(4):193–9.
Xu Q, Jin C, Jiang Y. Compare the flammability of two extruded polystyrene foams with micro-scale combustion calorimeter and cone calorimeter tests. J Therm Anal Calorim. 2017;127(3):2359–66.
Cogen JM, Lin TS, Lyon RE. Correlations between pyrolysis combustion flow calorimetry and conventional flammability tests with halogen-free flame-retardant polyolefin compounds. Fire Mater. 2009;33(1):33–50.
Lyon RE, Walters RN, Stoliarov SI. Thermal analysis of flammability. J Therm Anal Calorim. 2007;89(2):441.
Lin TS, Cogen JM, Lyon RE. Correlations between microscale combustion calorimetry and conventional flammability tests for flame retardant wire and cable compounds. In: Proceedings of the 56th IWCS conference, Lake Buena Vista, FL; 2007.
Xu Q, Jin C, Majlingova A, Restas A. Discuss the heat release capacity of polymer derived from microscale combustion calorimeter. J Therm Anal Calorim. 2017;133:1–9.
Hall G. Pearson’s correlation coefficient. http://www.hep.ph.ic.ac.uk/~hallg/UG_2015/Pearsons.pdf. Accessed 20 Mar 2018.
Zou KH, Tuncali K, Silverman SG. Correlation and simple linear regression. Radiology. 2003;227(3):617–28.
Xu Q, Majlingova A, Zachar M, Jin C, Jiang Y. Correlation analysis of cone calorimetry test data assessment of the procedure with tests of different polymers. J Therm Anal Calorim. 2012;110(1):65–70.
Ayoola BO, Balachandran R, Frank JH, Mastorakos E, Kaminski CF. Spatially resolved heat release rate measurements in turbulent premixed flames. Combust Flame. 2006;144(1–2):1–6.
Di Blasi C. Kinetic and heat transfer control in the slow and flash pyrolysis of solids. Ind Eng Chem Res. 1996;35(1):37–46.
Xu Q, Jin C, Hristov J, Griffin G, Jiang Y. The melt\shrink effect of low density thermoplastics insulates: cone calorimeter tests. Therm Sci. 2015;00:58.
Jiang L, He JJ, Sun JH. Sample width and thickness effects on upward flame spread over PMMA surface. J Hazard Mater. 2018;342:114–20.
An W, Jiang L, Sun J, Liew KM. Correlation analysis of sample thickness, heat flux, and cone calorimetry test data of polystyrene foam. J Therm Anal Calorim. 2015;119(1):229–38.
Mowrer FW. An analysis of effective thermal properties of thermally thick materials. Fire Saf J. 2005;40(5):395–410.
Delichatsios MA. Ignition times for thermally thick and intermediate conditions in flat and cylindrical geometries. Fire Saf Sci. 2000;6:233–44.
Acknowledgements
This research is supported by the National Natural Science Fund of China, No. 51776098 and the Fundamental Research Funds for the Central Universities, No. 30918015101.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mensah, R.A., Xu, Q., Asante-Okyere, S. et al. Correlation analysis of cone calorimetry and microscale combustion calorimetry experiments. J Therm Anal Calorim 136, 589–599 (2019). https://doi.org/10.1007/s10973-018-7661-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7661-5