Abstract
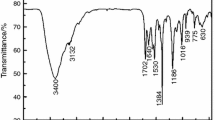
A new nitrogen-rich energetic salt of bis-1-methylimidazole 1H,1′H-5,5′-bistetrazole-1,1′-diolate salt, (1-M)2BTO, was synthesized and characterized (FT-IR, 1H NMR, 13C NMR, elemental analysis, and X-ray single-crystal diffraction). Results indicated that (1-M)2BTO crystallizes in the triclinic space group P-1. The thermal decomposition behavior of (1-M)2BTO was determined by differential scanning calorimetry (DSC) and thermogravimetric tandem infrared spectroscopy. The decomposition peak temperature of (1-M)2BTO was 530 K, which suggested that the salt is strong heat resistance. The apparent activation energies were 130.56 kJ mol−1 (Kissinger’s method) and 132.50 kJ mol−1 (Ozawa’s method), respectively. The enthalpy of formation for the salt was calculated as 917.3 kJ mol−1. The detonation velocity and detonation pressure of (1-M)2BTO were 7448 m s−1 and 20.7 GPa, respectively, using the Kamlet-Jacobs equation. Furthermore, the sensitivity test results showed that its impact sensitivity is greater than 50 J and friction sensitivity is 180 N, indicating that it has a lower sensitivity.










Similar content being viewed by others
References
Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP. Advances in science and technology of modern energetic materials: an overview. J Hazard Mater. 2008;151:289–305.
Cumming A. Energetic materials and the environment. Propellants Explos Pyrot. 2017;42:5–6.
Bachmann WE, Sheehan JC. A new method of preparing the high explosive RDX. J Am Chem Soc. 1949;71:1842–5.
Monteilrivera F, Paquet L, Halasz A, Montgomery MT, Hawari J. Reduction of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine by zerovalent iron: product distribution. Environ Sci and Technol. 2005;39:9725–31.
Simini M, Checkai RT, Kuperman RG, Phillips CT, Kolakowski JE, Kurnas CW, Sunahara GI. Reproduction and survival of Eisenia fetida in a sandy loam soil amended with the nitro-heterocyclic explosives RDX and HMX. The 7th international symposium on earthworm. Ecology. 2003;47:657–62.
Wang RH, Xu HY, Guo Y, Sa RJ, Shreeve JM. Bis[3-(5-nitroimino-1,2,4-triazolate)]-based energetic salts: synthesis and promising properties of a new family of high-density insensitive materials. J Am Chem Soc. 2010;132:11904–5.
Yin P, Zhang QH, Zhang JH, Parrish DA, Shreeve JM. N-trinitroethylamino functionalization of nitroimidazoles: a new strategy for high performance energetic materials. J Mater Chem A. 2013;1:7500–10.
Feng YY, Liu XY, Duan LQ, Yang Q, Wei Q, Xie G, Chen SP, Yang XW, Gao SL. In situ synthesized 3D heterometallic metal-organic framework (MOF) as a high-energy-density material shows high heat of detonation, good thermostability and insensitivity. Dalton Trans. 2015;44:2333–9.
He L, Tao GH, Parrish DA, Shreeve JM. Impact insensitive dinitromethanide salts. Chem Commun. 2013;49:10329–31.
Zhang WQ, Zhang JH, Deng MC, Qi XJ, Nie FD, Zhang QH. A promising high-energy-density material. Nat Commun. 2017;8:181.
Feng YG, Bi YG, Zhao WY, Zhang TL. Anionic metal-organic frameworks lead the way to eco-friendly high-energy-density materials. J Mater Chem A. 2016;4:7596–600.
Talawar MB, Sivabalan R, Mukundan T, Muthurajan H, Sikder AK, Gandhe BR, Rao AS. Environmentally compatible next generation green energetic materials (GEMs). J Hazard Mater. 2009;161:589–607.
Zhang JH, Zhang QH, Vo TT, Parrish DA, Shreeve JM. Energetic salts with π-stacking and hydrogen-bonding interactions lead the way to future energetic materials. J Am Chem Soc. 2015;137:1697–704.
Klapoetke TM, Preimesser A, Stierstorfer J. Energetic derivatives of 4,4′,5,5′-tetranitro-2, 2′-bisimidazole (TNBI). Z Anorg Allg Chem. 2012;638:1278–86.
Klapoetke TM, Martin FA, Mayr NT, Stierstorfer J. Synthesis and characterization of 3,5-diamino-1,2,4-triazolium dinitramide. Z Anorg Allg Chem. 2010;636:2555–64.
Tselinskii IV, Mel’nikova SF, Romanova TV. Synthesis and reactivity of carbohydroximoyl azides: I. Aliphatic and aromatic carbohydroximoyl azides and 5-substituted 1-hydroxytetrazoles based thereon. Russ J Org Chem. 2001;37:430–6.
Fischer N, Klapoetke TM, Reymann M, Stierstorfer J. Nitrogen-rich salts of 1H,1′H-5,5′-bitetrazole-1,1′-diol: energetic materials with high thermal stability. Eur J Inorg Chem. 2013;2013:2167–80.
Fischer N, Fischer D, Klapoetke TM, Piercey DG, Stierstorfer J. Pushing the limits of energetic materials: the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J Mater Chem. 2012;22:20418–22.
Shang Y, Jin B, Liu QQ, Peng RF, Guo ZC, Zhang QC. Synthesis, thermal behavior, and energetic properties of diuronium 1H,1′H-5,5′-bistetrazole-1,1′-diolate salt. J Mol Struct. 2017;1133:519–25.
Shang Y, Jin B, Peng RF, Guo ZC, Liu QQ, Zhao J, Zhang QC. Nitrogen-rich energetic salts of 1H,1′H-5,5′-bistetrazole-1,1′-diolate: synthesis, characterization, and thermal behaviors. RSC Adv. 2016;6:48590–8.
Zhang ZB, Yin L, Yin X, Zhang JG. Preparation, crystal and properties of nitrogen-rich energetic salt of bis(semicarbazide)5,5′-bitetrazole-1,1′-diolate. Crystals. 2016;6:76075–83.
Fischer N, Izsák D, Klapoetke TM, Rappenglüeck S, Stierstorfer J. Nitrogen-rich 5,5′-bistetrazolates and their potential use in propellant systems: a comprehensive study. Chem Eur J. 2012;18:4051–62.
Huang HF, Zhou ZM, Liang LX, Song JH, Wang K, Cao D, Bian CM, Sun WW, Xue M. Nitrogen-rich energetic dianionic salts of 3,4-bis(1H-5-tetrazolyl)furoxan with excellent thermal stability. Z Anorg Allg Chem. 2012;638:392–400.
Niu H, Chen SS, Jin SH, Li LJ, Jing BC, Jiang ZM, Ji JW, Shu QH. Thermolysis, nonisothermal decomposition kinetics, calculated detonation velocity and safety assessment of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J Therm Anal Calorim. 2016;126:473–80.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Doyle C. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92.
Ozawa T. A new method of analyzing thermogravimetric data. B Chem Soc Jpn. 1965;38:1881–6.
Li ZM, Xie SH, Zhang JG, Feng JL, Wang K, Zhang TL. Two high nitrogen content energetic compounds: 3,6-diguanidino-1,2,4,5-tetrazine and its diperchlorate. J Chem Eng Data. 2012;57:729–36.
Kamlet MJ, Jacobs SJ. Chemistry of detonations. I. A simple method for calculating detonation properties of C-H-N-O explosives. J Chem Phys. 1968;48:23–35.
Jenkins HDB, Tudela D, Glasser L. Lattice potential energy estimation for complex ionic salts from density measurements. Inorg Chem. 2002;41:2364–7.
Sućeska M. Calculation of the detonation properties of C-H-N-O explosives. Propellants, Explos, Pyrotech. 1991;16:197–202.
Verevkin SP, Zaitsau DH, Emel’yanenko VN, Paulechka YU, Blokhin AV, Bazyleva AB, Kabo GJ. Thermodynamics of ionic liquids precursors: 1-methylimidazole. J Phys Chem B. 2011;115:4404–11.
Tang YX, Gao HX, Parrish DA, Shreeve JM. 1,2,4-Triazole links and N-azo bridges yield energetic compounds. Chem Eur J. 2015;21:11401–7.
Gao HX, Shreeve JM. Azole-based energetic salts. Chem Rev. 2011;111:7377–436.
Acknowledgements
This work was supported by the financial support received from the Science Challenge Project (Project No. TZ2018004), the National Natural Science Foundation of China (Project No. 51372211), the China Academy of Engineering Physics Research Institute (Project No. 18zh0079) and Open Project of State Key Laboratory Cultivation Base for Nonmetal Composites and Functional (Project No. 14tdfk05).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Luo, L., Jin, B., Peng, R. et al. Preparation and characterization of nitrogen-rich bis-1-methylimidazole1H,1′H-5,5′-bistetrazole-1,1′-diolate energetic salt. J Therm Anal Calorim 135, 3005–3013 (2019). https://doi.org/10.1007/s10973-018-7481-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7481-7