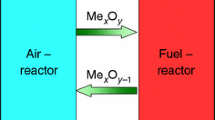
Abstract
Alumina-supported Fe2O3 oxygen carrier material (OCM) system is among the most promising OCM systems for solid and gaseous fuel CLC. This work utilizes a comprehensive thermogravimetric and thermodynamic equilibrium approach to redox and CLOU performance, oxygen transfer capacity, reduction rate and sulfur tolerance of the Fe2O3 impregnated on Al2O3 OCM. Thermodynamic evaluations reveal that the beneficial composition range lies in a wide range of 7.5–34% molar Fe2O3 ratios. This is the range at which aluminum-rich corundum phase, i.e., (Al, Fe)2O3, remains stable throughout the oxidizing to very reducing oxygen partial pressures in fuel reactor. The experimental system in this study contains 20 mass% Fe2O3, i.e., XFe = 13.8% molar which lies well within this interval. Deep redox cycle experiments confirm the thermodynamic modeling and during the long residence time of this experiment, the sample is almost fully reduced and exhibits its thermodynamic redox oxygen capacity of close to 1.5 mass%. Extension of the deep redox cycles to 15 cycles induces no performance deterioration in terms of capacity, rate of reduction or morphological failure. The redox experiment under sour reducing gas indicates no H2S poisoning for the 20 mass% Fe2O3 supported on Al2O3 OCM. The findings that this system is not affected with the H2S content of the gas, and the prediction of the SO2 release from the fuel reactor is in good agreement with our recent reactor testing findings available in the literature.









Similar content being viewed by others
References
Fan LS. Chemical looping systems for fossil energy conversions. Hoboken: Wiley; 2011.
Hossain MM, de Lasa HI. Chemical-looping combustion (CLC) for inherent separations: a review. Chem Eng Sci. 2008;63:4433–51.
Erlach B, Schmidt M, Tsatsaronis G. Comparison of carbon capture IGCC with pre-combustion decarbonisation and with chemical-looping combustion. Energy. 2011;36:3804–15.
Adanez J, de Diego LF, Garcia-Labiano F, Gayan P, Abad A, Palacios JM. Selection of oxygen carriers for chemical-looping combustion. Energy Fuels. 2004;18:371–7.
Abad A, Adánez J, García-Labiano F, de Diego LF, Gayán P, Celaya J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion. Chem Eng Sci. 2007;62:533–49.
Larring Y, Braley C, Pishahang M, Andreassen KA, Bredesen R. Evaluation of a mixed Fe–Mn oxide system for chemical looping combustion. Energy Fuels. 2015;29:3438–45.
Larring Y, Pishahang M, Sunding MF, Tsakalakis K. Fe–Mn based minerals with remarkable redox characteristics for chemical looping combustion. Fuel. 2015;159:169–78.
Moghtaderi B, Song H. Reduction properties of physically mixed metallic oxide oxygen carriers in chemical looping combustion. Energy Fuels. 2010;24:5359–68.
Mukherjee S, Kumar P, Yang A, Fennell P. A systematic investigation of the performance of copper-, cobalt-, iron-, manganese- and nickel-based oxygen carriers for chemical looping combustion technology through simulation models. Chem Eng Sci. 2015;130:79–91.
Zhang S, Rajendran S, Henderson S, Zeng D, Xiao R, Bhattacharya S. Use of pyrite cinder as an iron-based oxygen carrier in coal-fueled chemical looping combustion. Energy Fuels. 2015;29:2645–55.
Abbasi M, Farniaei M, Rahimpour MR, Shariati A. Simultaneous syngas production with different H2/CO ratio in a multi-tubular methane steam and dry reformer by utilizing of CLC. J Energy Chem. 2015;24:54–64.
Galvita VV, Poelman H, Bliznuk V, Detavernier C, Marin GB. CeO2-Modified Fe2O3 for CO2 utilization via chemical looping. Ind Eng Chem Res. 2013;52:8416–26.
Zhu X, Li K, Wei Y, Wang H, Sun L. Chemical-looping steam methane reforming over a CeO2–Fe2O3 oxygen carrier: evolution of its structure and reducibility. Energy Fuels. 2014;28:754–60.
Ismail M, Liu W, Scott SA. The performance of Fe2O3–CaO oxygen carriers and the interaction of iron oxides with CaO during chemical looping combustion and H2 production. Energy Procedia. 2014;63:87–97.
Liu W, Dennis JS, Scott SA. The effect of addition of ZrO2 to Fe2O3 for hydrogen production by chemical looping. Ind Eng Chem Res. 2012;51:16597–609.
Azimi G, Leion H, Mattisson T, Ryden M, Snijkers F, Lyngfelt A. Mn–Fe oxides with support of MgAl2O4, CeO2, ZrO2 and Y2O3–ZrO2 for chemical-looping combustion and chemical-looping with oxygen uncoupling. Ind Eng Chem Res. 2014;53:10358–65.
Qin W, Chen Q, Wang Y, Dong C, Zhang J, Li W, Yang Y. Theoretical study of oxidation–reduction reaction of Fe2O3 supported on MgO during chemical looping combustion. Appl Surf Sci. 2013;266:350–4.
Gu Z, Li K, Wang H, Wei Y, Yan D, Qiao T. Syngas production from methane over CeO2–Fe2O3 mixed oxides using a chemical-looping method. Kinet Catal. 2013;54:326–33.
Jerndal E, Mattisson T, Lyngfelt A. Thermal analysis of chemical-looping combustion. Chem Eng Res Des. 2006;84:795–806.
Cabello A, Gayan P, Pans MA, Dueso C, Garcia-Labiano F, Abad A, de Diego LF, Adanez J. Evaluation of a highly reactive and sulfur resistant synthetic Fe-based oxygen carrier for CLC using gaseous fuels. Energy Procedia. 2013;37:580–7.
Gayán P, Pans MA, Ortiz M, Abad A, de Diego LF, García-Labiano F, Adánez J. Testing of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for a SR–CLC system in a continuous CLC unit. Fuel Process Technol. 2012;96:37–47.
Zhang Y, Doroodchi E, Moghtaderi B. Comprehensive study of Fe2O3/Al2O3 reduction with ultralow concentration methane under conditions pertinent to chemical looping combustion. Energy Fuels. 2015;29:1951–60.
Zhang Y, Doroodchi E, Moghtaderi B. Reduction kinetics of Fe2O3/Al2O3 by ultralow concentration methane under conditions pertinent to chemical looping combustion. Energy Fuels. 2015;29:337–45.
Ishida M, Takeshita K, Suzuki K, Ohba T. Application of Fe2O3–Al2O3 composite particles as solid looping material of the chemical-loop combustor. Energy Fuels. 2015;19:2514–8.
Zhang J, Guo Q, Liu Y, Cheng Y. Preparation and characterization of Fe2O3/Al2O3 using the solution combustion approach for chemical looping combustion. Ind Eng Chem Res. 2012;51:12773–81.
Chiu PC, Ku Y, Wu YL, Wu HC, Kuo TL. Characterization and evaluation of prepared Fe2O3/Al2O3 oxygen carriers for chemical looping process. Aerosol Air Qual Res. 2014;14:981–90.
Mei D, Abad A, Zhao H, Adánez J, Zheng C. On a highly reactive Fe2O3/Al2O3 oxygen carrier for in situ gasification chemical looping combustion. Energy Fuels. 2014;28:7043–52.
Guo L, Zhao HB, Ma JC, Mei D, Zheng C. Comparison of large-scale production methods of Fe2O3/Al2O3 oxygen carriers for chemical-looping combustion. Chem Eng Technol. 2014;37:1211–9.
de Diego LF, García-Labiano F, Gayán P, Abad A, Cabello A, Adánez J, Sprachmann G. Performance of Cu- and Fe-based oxygen carriers in a 500 Wth CLC unit for sour gas combustion with high H2S content. Int J Greenh Gas Control. 2014;28:168–79.
Hafizi A, Rahimpour MR, Hassanajili S. Calcium promoted Fe/Al2O3 oxygen carrier for hydrogen production via cyclic chemical looping steam methane reforming process. Int J Hydrog Energy. 2015;40:16159–68.
Wang Y, Wang X, Hua X, Zhao C, Wang W. The reduction mechanism and kinetics of Fe2O3 by hydrogen for chemical-looping hydrogen generation. J Therm Anal Calorim. 2014;129:1831–8.
Abad A, García-Labiano F, de Diego LF, Gayán P, Adánez J. Reduction kinetics of Cu-, Ni-, and Fe-based oxygen carriers using syngas (CO + H2) for chemical-looping combustion. Energy Fuels. 2017;21:1843–53.
Cabello A, Abad A, García-Labiano F, Gayán P, de Diego L, Adánez J. Kinetic determination of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for use in gas-fueled chemical looping combustion. Chem Eng J. 2014;258:265–80.
Cabello A, Dueso C, García-Labiano F, Gayán P, Abad A, de Diego L, Adánez J. Performance of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier with CH4 and H2S in a 500 Wth CLC unit. Fuel. 2014;121:117–25.
Shafiefarhood A, Stewart A, Li F. Iron-containing mixed-oxide composites as oxygen carriers for Chemical Looping with Oxygen Uncoupling (CLOU). Fuel. 2015;139:1–10.
Acknowledgements
The work presented in this article is conducted as part of the European Union Seventh Framework Programme (FP7/2007-2013) under Grant Agreement No. 608571 (Project acronym SUCCESS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pishahang, M., Larring, Y., Adánez, J. et al. Fe2O3–Al2O3 oxygen carrier materials for chemical looping combustion, a redox thermodynamic and thermogravimetric evaluation in the presence of H2S. J Therm Anal Calorim 134, 1739–1748 (2018). https://doi.org/10.1007/s10973-018-7422-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7422-5