Abstract
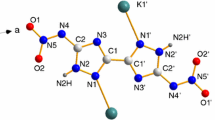
N-trinitromethyl-4,5-dicyano-2H-1,2,3-triazole was readily synthesized from 4,5-dicyano-2H-1,2,3-triazole. Its crystal structure was obtained for the first time and its crystalline density in 296 K was 1.729 g cm−3. It shows high nitrogen and oxygen content up to 77.6%, high calculated solid heat of formation (564 kJ mol−1), and superior detonation pressure and detonation velocity (D = 8619 m s−1, P = 30.8 GPa). This new hydrogen-absent explosive shows high impact and friction sensitivities (IS: 1.25 J, FS: 32 N), which is lower than commercial primary explosive 2-diazonium-4,6-dinitrophenol (DDNP) (IS: 1 J, FS: 5 N). The relationship between intermolecular interaction and sensitivity as well as thermal stability of the title compound was investigated by Hirshfeld surface analysis and fingerprint plot. Its thermodynamic properties were studied by non-isothermal kinetic methods based on the results of differential scanning calorimeter. It is interesting that apparent activation energy (Ea) at Tp1 (210.89–214.17 kJ mol−1) is higher than those at Tp2 (133.90–134.87 kJ mol−1). In addition, gaseous product of this new energetic compound was analyzed by the rapid scanning Fourier transform infrared spectroscopy from 20 to 200 °C and its detonation products was theoretically predicted. Based on the decomposition products, its decomposition mechanism was discussed under inert atmosphere. It is undoubted that these significant physicochemical properties make N-trinitromethyl-4,5-dicyano-2H-1,2,3-triazole a potential hydrogen-absent primary explosive.














Similar content being viewed by others
References
Yin P, Zhang Q, Shreeve JM. Dancing with energetic nitrogen atoms: versatile N-functionalization strategies for N-heterocyclic frameworks in high energy density materials. Acc Chem Res. 2016;49:4–16.
Kettner MA, Karaghiosoff K, Klapötke TM, Sućeska M, Wunder S. 3,3′-Bi(1,2,4-oxadiazoles) featuring the fluorodinitromethyl and trinitromethyl group. Chem Eur J. 2014;20:1–11.
Yu Q, Yin P, Zhang J, He C, Imler GH, Parrish DA, Shreeve JM. Pushing the limits of oxygen balance in 1,3,4-oxadiazoles. J Am Chem Soc. 2017;139:8816–9.
Tian J, Xiong H, Lin Q, Cheng G, Yang H. Energetic compounds featuring bi(1,3,4-oxadiazole): a new family of insensitive energetic materials. New J Chem. 2017;41:1918–24.
Ma Q, Gu H, Huang J, Nie F, Fan G, Liao L, Yang W. Formation of trinitromethyl functionalized 1,2,4-triazole-based energetic ionic salts and a zweitterionic salt directed by an intermolecular and intramolecular metathesis strategy. New J Chem. 2018;42:2376–80.
He C, Shreeve JM. Energetic materials with promising properties: synthesis and characterization of 4,4′-bis(5-nitro-1,2,3-2H-triazole) derivatives. Angew Chem Int Ed. 2015;51:1–6.
Zhang Y, Du Z, Han Z, Yao Q, Hu Z. Synthesis and thermal analysis of 2-methyl-4,5-dicyano-2H-1,2,3-triazole. J Therm Anal Calorim. 2016;124:529–37.
Dippold AA, Izsák D, Klapötke TM, Pflüger C. Combining the advantages of tetrazoles and 1,2,3-triazoles:4,5-bis(tetrazol-5-yl)-1,2,3-triazole,4,5-bis(1-hydroxytetrazol-5-yl)-1,2,3-triazole, and their energetic derivatives. Chem Eur J. 2016;22:1768–78.
Dalinger IL, Vatsadze IA, Shkineva TK, Kormanov AV, Struchkova DI, Suponitsky KY, Bragin AA, Monogarov KA, Sinditskii VP, Sheremetev AB. Novel highly energetic pyrazoles: N-trinitromethyl-substituted nitropyrazoles. Chem Asian J. 2015;10:1987–96.
Crawford M-J, Karaghiosoff K, Klapötke TM, Martin FA. Synthesis and characterization of 4,5-dicyano-2H-1,2,3-triazole and its sodium, ammonium, and guanidinium salts. Inorg Chem. 2009;48:1731–43.
Ma Q, Gu H, Lu H, Liao L, Huang J, Fan G, Li J, Liu D. Synthesis of 5,6-di(2-fluoro-2,2-dinitroethoxy)-2,3-dicyanopyrazine by one-step nucleophilic substitution and its energetic properties. ChemistrySelect. 2017;2:4567–71.
Wang N, Chen B, Ou Y. Review on benzofuroxan system compounds. Propellants Explos Pyrotech. 1994;19:145–8.
Thottempudi V, Forohor F, Parrish DA, Shreeve JM. Tris(triazolo)benzene and its derivatives: high-density energetic materials. Angew Chem Int Ed. 2012;51:9881–5.
Spackman MA, Jayatilaka D. Hirshfeld surface analysis. Cryst Eng Commun. 2009;11:19–32.
Spackman MA, McKinnon JJ. Fingerprinting intermolecular interactions in molecular crystals. Cryst Eng Commun. 2002;4:378–92.
Yan Q-L, Zeman S, Zhang J, He P, Musil T, Bartoškova M. Multi-stage decomposition of 5-aminotetrazole derivatives: kinetics and reaction channels for the rate-limiting steps. Phys Chem Chem Phys. 2014;16:24282–91.
Ma Q, Lu H, Liao L, Chen Y, Cheng B, Fan G, Huang J. Synthesis and thermal decomposition performance of 3,6,7-triamino-7H-s-triazolo[5,1-c]-s-triazole. J Therm Anal Calorim. 2017;127:2517–29.
Ma Y, Zhang A, Xue X, Jiang D, Zhu Y, Zhang C. Crystal packing of impact-sensitive high-energy explosives. Cryst Growth Des. 2014;14:6101–14.
Wei X, Ma Y, Long X, Zhang C. A strategy developed from the observed energetic-energetic cocrystals of BTF: cocrystallizing and stabilizing energetic hydrogen-free molecules with hydrogenous energetic coformer molecules. Cryst Eng Commun. 2015;17:7150–9.
Yan Q-L, Zeman S. Theoretical evaluation of sensitivity and thermal stability for high explosives based on quantum chemistry methods: a brief review. Int J Quantum Chem. 2013;113:1049–61.
Zohari N, Keshavarz MH, Seyedsadjadi SA. A link between impact sensitivity of energetic compounds and their activation energies of thermal decomposition. J Therm Anal Calorim. 2014;117:423–32.
Liu Y, Jiang YT, Zhang TL, Feng CG, Yang L. Thermal kinetic performance and storage life analysis of a series of high-energy and green energetic materials. J Therm Anal Calorim. 2015;119:659–70.
Ma Q, Lu H, Qu Y, Liao L, Li J, Fan G, Chen Y. A facile synthesis of 3,3′-dinitro-5,5′-diamino-bi-1,2,4-triazole and a study of its thermal decomposition. Cent Eur J Energy Mater. 2017;14:281–95.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1957;38:1881–6.
Boswell PG. Calculation of activation energies using a modified Kissinger method. J Therm Anal Calorim. 1980;18:353–6.
Zhang T, Hu R, Xie Y, Li F. The estimation of critical temperatures of thermal explosion for energetic materials using non-isothermal DSC. Thermochim Acta. 1994;244:171–6.
Pourmortazavi SM, Nasrabadi MR, Kohsari I, Hajimirsadeghi SS. Non-isothermal kinetic studies on thermal decomposition of energetic materials KNF and NTO. J Therm Anal Calorim. 2012;110:857–63.
Sućeska M. Evaluation of detonation energy from EXPLO5 computer code results. Propellants Explos Pyrotech. 1999;24:280–5.
Politzer P, Murray JS, Grice ME, Desalvo M, Miller M. Calculation of heats of sublimation and solid phase heats of formation. Mol Phys. 1997;91:923–8.
Ma Q, Jiang T, Zhang X, Fan G, Wang J, Huang J. Theoretical investigations on 4,4′,5,5′-tetranitro-2,2′-1H,1′H-2,2′-biimidazole derivatives as potential nitrogen-rich high energy materials. J Phys Org Chem. 2015;28:31–9.
Klapötke TM, Preimesser A, Stierstorfer J. Synthesis and energetic properties of 4-diazo-2,6-dinitrophenol and 6-diazo-3-hydroxy-2,4-dinitrophenol. Eur J Org Chem. 2015;2015:4311–5.
Acknowledgements
The support of the National Natural Science Foundation of China (Nos. 11402237 and 11302200), the Science and Technology Development Funds of CAEP (No. 2015B0302055), and the NSAF Foundation of National Natural Science Foundation of China and China Academy of Engineering Physics (No. U1530262) are gratefully acknowledged. We are indebted to Mrs. Lin Wang for considerable assistance with RS-FTIR test.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, X., Li, J., Zhang, G. et al. Synthesis and thermal decomposition behavior of nitrogen- and oxygen-rich energetic material N-trinitromethyl-4,5-dicyano-2H-1,2,3-triazole. J Therm Anal Calorim 135, 2317–2328 (2019). https://doi.org/10.1007/s10973-018-7390-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7390-9