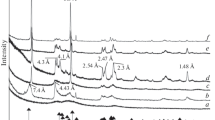
Abstract
Systematic modification of three structurally different minerals (zeolite, mica, and vermiculite) was carried out with the aim of determining the modification mechanism and exposing the hydrophobic surface that can be used as a sorbent for many organic compounds. Mechanism of modification with cationic surfactant depends strongly on the mineral type. In order to identify the influence of aluminosilicates structural differences on the modification process, adsorption experiments with organic matter and water vapor, supplemented with the DTA/TG analysis, were performed. The cation exchange capacity (CEC) value was 1454 > 560 > 28 meq kg−1 for zeolite (clinoptilolite), vermiculite, and mica (muscovite), respectively. Despite its CEC value, vermiculite adsorbed three times the amount of organic matter than did clinoptilolite due to the porous structure of zeolite, which acted to limit the adsorption only on the external exchangeable cations. If the loading amount is equal to the CEC or the external cation exchange capacity for clinoptilolite (ECEC ≈ 10% CEC), the monolayer will form while mineral surface will have hydrophobic character. Only one active center exists at the surface of the clinoptilolite that was identified by DTA curves with a sharp and defined peak around 300 °C and by the mass loss at the TG diagrams. Two significant and equal active centers were observed in vermiculite, one for the exchange of the surface cations and the other for the interlayer cations and H2O molecules. Muscovite CEC is negligible, and due to the absence of any other functional groups, the modification of this mineral was impossible.





Similar content being viewed by others
References
Wan Ngah WS, Isa IM. Comparison study of copper ion adsorption on chitosan, Dowex A-1, and Zerolit 225. J Appl Polym Sci. 1998;67:1067–70.
Crini G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci. 2005;30:38–70.
Ajmal M, Rao R, Ahmad J. Adsorption studies on Citrus reticulata (fruit peel of orange): removal and recovery of Ni(II) from electroplating wastewater. J Hazard Mater. 2000;79:117–31.
Amana T, Kazi AA, Sabri MU, Banoa Q. Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surf B: Biointerfaces. 2008;63:116–21.
Kurniawan TA, Chan GYS, Lo W, Babel S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ. 2006;366:409–26.
Ahluwalia SS, Goyal D. Biosorption of Cu(II) from aqueous solution by Fucus serratus: surface characterization and sorption mechanisms. Bioresour Technol. 2008;99:6150–5.
Šćiban M, Radetić B, Kevrešanin Ž, Klašnja M. Adsorption of heavy metals from electroplating wastewater by wood sawdust. Bioresour Technol. 2007;98:402–9.
Carmody O, Frost R, Xi Y, Kokot S. Adsorption of hydrocarbons on organo-clays—implications for oil spill remediation. J Colloid Interface Sci. 2007;305:17–24.
Wang D, Liu Q, Cheng H, Zhang S, Zuo X. Effect of reaction temperature on intercalation of octyltrimethylammonium chloride into kaolinite. J Therm Anal Calorim. 2017;128(3):1555–64.
Chun Y, Sheng GY, Boyd SA. Sorptive characteristics of tetraalkylammonium-exchanged smectite clays. Clay Clay Miner. 2003;51:451–8.
Boyd SA, Chiou CT, Mortland MM. Sorption characteristics of organic compounds on hexadecyltrimethylammoniumsmectite. Soil Sci Soc Am J. 1998;52:652–60.
Boyd SA, Jaynes WF, Ross BS. Immobilization of organic contaminants by organo-clays. Application to soil restoration and hazardous waste containment. In: Baker RS, editor. Organic substances and sediments in water. Chelsea: Lewis Publishers; 1989. p. 181–200.
Yariv S, Borisover M, Lapides I. Few introducing comments on the thermal analysis of organoclays. J Therm Anal Calorim. 2011;105:897–906.
Lemić J, Kovačević D, Tomašević-Čanović M, Kovačević D, Stanić T, Pfend R. Removal of atrazine, lindane and diazinone from water by organo-zeolites. Water Res. 2006;40:1079–85.
Kovacević D, Lemić J, Damjanović M, Petronijević R, Janaćković Đ, Stanić T. Fenitrothion adsorption—desorption on organo—minerals. Appl Clay Sci. 2011;52:109–14.
Lemić J, Tomašević-Čanović M, Adamović M, Kovačević D, Milićević S. Competitive adsorption of polycyclic aromatic hydrocarbons on organo-zeolites. Microporous Mesoporous Mater. 2007;105:317–23.
Milićević S, Matović LJ, Petrović Đ, Đukić A, Milošević V, Đokić D, Kumrić K. Surfactant modification and adsorption properties of clinoptilolite for the removal of pertechnetate from aqueous solutions. J Radioanal Nucl Chem. 2016;310:805–15.
Gottardi G, Galli E. General information on zeolites. Natural Zeolites. Berlin: Springer; 1985. p. 1–34.
Doula M. Removal of Mn2 + ions from drinking water by using clinoptilolite and a clinoptilolite-Fe oxide system. Water Res. 2006;40:3167–76.
Schulze DG, Dixon JB, Weed SB. Minerals in soil environments. Medison: SSSA; 1989.
Grimshaw RW. The chemistry and physics of clays. London: Ernest Benn Limited; 1971.
Jansson SO, Modin R, Schill G. Two phase titration of organic ammonium ions with lauryl sulphate and methyl yellow as indicator. Talanta. 1974;21(9):905–18.
Dultza S, Riebeb B, Bunnenbergb C. Temperature effects on iodine adsorption on organo-clay minerals II. Structural effects. Appl Clay Sci. 2005;28:17–30.
Uchida Y, Hishiya S, Fujii N, Kohmura K, Nakayama T, Tanaka H, Kikkawa T. Effect of moisture adsorption on the properties of porous-silica ultralow-k films. Microelectron Eng. 2006;83:2126–9.
De La Calle C, Suquet H, Pons C. Stacking order in a 14.30-A Mg-vermiculite. Clays Clay Miner. 1988;36(6):481–90.
Yariv S. Wettability of clay minerals. In: Schrader ME, Loeb G, editors. Modern approach to wettability. New York: Plenum Press; 1992. p. 279–326.
Yariv S. The role of charcoal on DTA curves of organo-clay complexes: an overview. Appl Clay Sci. 2004;24:225–36.
Acknowledgements
This research has been financed by the Ministry of Education, Science, and Technological Development of Republic of Serbia as part of the Project TR 033007. The authors would like to express their gratitude for this support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milićević, S., Martinović, S., Milošević, V. et al. Differences in coating mechanism of structurally different aluminosilicates observed through the thermal analysis. J Therm Anal Calorim 134, 1011–1019 (2018). https://doi.org/10.1007/s10973-018-7351-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7351-3