Abstract
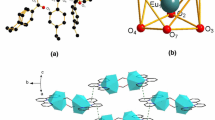
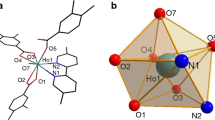
The novel complex [Nd(3,4,5-TEOBA)3phen]2(3,4,5-TEOBA = 3,4,5-triethoxybenzoate; phen = 1,10-phenanthroline) has been synthesized and characterized by elemental analysis, IR spectra, single-crystal X-ray diffraction, and thermogravimetry/differential scanning calorimetry-Fourier transform infrared (TG/DSC-FTIR) technology. Single-crystal X-ray diffraction data show the complex has two center metals, and each center is coordinated by seven oxygen atoms and two nitrogen atoms to form a distorted monocapped square antiprism geometry. The binuclear molecular skeleton connects together to form a 1D chain via stacking π–π interactions. The thermal decomposition mechanism of the title complex has been studied by TG/DSC-FTIR technology. The low-temperature heat capacity of the complex has been measured using a physical property measurement system in the temperature range from 1.9 to 300 K. The heat capacity was fitted to a series of theoretical and empirical models, and the thermodynamic functions were calculated based on the curve fitting.







Similar content being viewed by others
References
Onodera H, Nakajima A, Nakanishi T, Fushimi K, Hasegawa Y. Thermostable Eu(III)-nanorod luminophores with effective photosensitized energy transfer. J Alloy Compd. 2015;648:651–7.
Li QF, Yue D, Ge GW, Du XD, Gong YC, Ling WZ, Hao JH. Water-soluble Tb3+ and Eu3+ complexes based on task-specific ionic liquid ligands and their application in luminescent poly(vinyl alcohol) films. Dalton T. 2015;44:16810–7.
Wang D, Luo Z, Wang DJ, Fan L, Yin GD. Synthesis and photoluminescent properties of Eu(III) complexes with fluorinated β-diketone and nitrogen heterocyclic ligands. Dyes Pigments. 2016;132:398–404.
Sukhikh TS, Bashirov DA, Kolybalov DS, Andreeva AY, Smolentsev AI, Kuratiev NV, Burilov VA, Mustafina AR, Kozlova SG, Konchenk SN. Synthesis, luminescent and magnetic properties of new tetranuclear lanthanide complexes with 4-hydroxy-2,1,3-benzothiadiazolate and dibenzoylmethanide ligands. Polyhedron. 2017;124:139–44.
Wang Q, Fan Y, Song TY, Xu JN, Wang J, Chai J, Liu YL, Wang L, Zhang LR. In situ synthesis of a series of lanthanide coordination polymers based on N-heterocyclic carboxylate ligands: crystal structure and luminescence. Inorg Chim Acta. 2015;438:128–34.
Matias FRM, da Silva VMF, Nunes RS, Luiz JM. Synthesis, characterization and thermal behavior of some trivalent lanthanide 4-amino-benzenesulfonate salts. J Therm Anal Calorim. 2017;130:2185–90.
Behzad SK, Najafi E, Amini MM, Ng SW. Sonochemical synthesis of a nanoscale complex of neodymium(III) and 8-hydroxy-2-methylquinoline: spectroscopic, photoluminescence, and thermal analysis. Monatsh Chem. 2015;146:571–80.
Wang Y, Zhao QQ, Ren N, Zhang JJ, Geng LN, Wang SP. Crystal structures, thermal properties, and luminescent properties of two novel mononuclear lanthanide compounds with 2,4-dichlorobenzoic acid and 2,2′:6′,2″-terpyridine. J Therm Anal Calorim. 2016;126:1703–12.
Zhang S, Yang Y, Xia ZQ, Liu XY, Yang Q, Wei Q, Xie G, Chen SP, Gao SL. Eu-MOFs with 2-(4-carboxyphenyl)imidazo[4,5-f]-1,10-phenanthroline and ditopic carboxylates as coligands: synthesis, structure, high thermostability, and luminescence properties. Inorg Chem. 2014;53(20):10952–63.
Bai FH, Wen D, Gao YJ, Zhang LP, Suo YJ, Su HQ. A series of supramolecular complexes constructed from discrete lanthanide dinuclear butterfly-shaped clusters: syntheses, structures and properties. Inorg Chem Commun. 2017;86:70–3.
Fu R, Hu S, Wu X. New 3D lanthanide phosphonates: syntheses, crystal structure, thermal stability, luminescence, and magnetism. Cryst Growth Des. 2014;14:6197–204.
Pitchaimani P, Lo KM, Elango KP. Synthesis, crystal structures, luminescence properties and catalytic application of lanthanide(III) piperidine dithiocarbamate complexes. Polyhedron. 2015;93:8–16.
Alizadeh R, Amani V. Syntheses, crystal structures, and photoluminescence of three cadmium(II) coordination complexes based on bipyridine ligands with different positioned methyl substituents. Inorg Chim Acta. 2016;443:151–9.
Wang ZN, Xu XT, Lv X, Bai FY, Liu SQ, Xing YH. Synthesis, crystal structure, fluorescence and antimicrobial activity of a series of rare-earth complexes based on indolebutyric acid. RSC Adv. 2015;5:104263–74.
Luo GH, Gao XH, Pan L, Lv XC, Tan ZC. Low-temperature molar heat capacities and thermodynamic properties of a new rare earth complex Er2(μ2-Gly)6(H2O)4·Na2(ClO4)8(H2O)2·4H2O. J Therm Aanl Calorm. 2016;126:871–9.
Shi Q, Snow CL, Boerio-Goates J, Woodfield BF. Accurate heat capacity measurements on powdered samples using a quantum design physical property measurement system. J Chem Thermodyn. 2010;42:1107–15.
Shi Q, Boerio-Goates J, Woodfield BF. An improved technique for accurate heat capacity measurements on powdered samples using a commercial relaxation calorimeter. J Chem Thermodyn. 2011;43:1263–9.
Zhao QQ, Ren N, Zhang JJ, Geng LN, Wang SP, Shi SK. Three novel Ho(III) complexes with different auxiliary ligands: synthesis, crystal structures and thermal properties. Polyhedron. 2017;132:78–89.
Wang Y, Shen PP, Ren N, Zhang JJ, Geng LN, Wang SP, Shi SK. A series of lanthanide compounds with different N-donor ligands: synthesis, structures, thermal properties and luminescence behaviors. RSC Adv. 2016;6:70770–80.
Zhang YY, Ren N, Xu SL, Zhang JJ, Zhang DH. A series of binuclear lanthanide(III) complexes: crystallography, antimicrobial activity and thermochemistry properties studies. J Mol Struct. 2015;1081:413–25.
Zhang XY, Xue B, Cheng Z, Tan ZC, Shi Q. Low temperature heat capacities of uracil and 5-bromouracil. Acta Phys Chim Sin. 2015;31:412–8.
Acknowledgements
The research work was supported by the National Natura Science Foundation of China (No. 21473049) and the Natural Science Foundation of Hebei Province (No. B2016205207). Science and technology program of Handan (No. 1621202043-2).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qi, XX., Shi, Q., Ren, N. et al. A neodymium(III) complex with 3, 4, 5 - triethoxybenzoic acid and 1,10-phenanthroline . J Therm Anal Calorim 135, 2583–2590 (2019). https://doi.org/10.1007/s10973-018-7269-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7269-9