Abstract
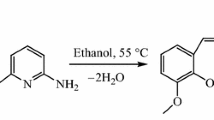
In this study, a new Schiff base ligand [2-{[4-amino-5-(3,4,5-trimethoxy-benzyl)-pyrimidin-2-ylimino]-methyl}-6-methoxy-phenol, C22H24N4O5] and its bismuth(III) complex [BiC22H22N4O5Cl] were synthesized using o-vanillin and trimethoprim in anhydrous solvents. Chemical analysis, elemental analysis, spectrum analysis and TG–DSC analysis were employed to characterize the compositions and structures of the two new compounds. In particular, the metabolic thermogenic curves of Schizosaccharomyces pombe (S. pombe) and Helicobacter pylori (H. pylori) cells treated by the two new compounds at different concentrations were monitored by an isothermal microcalorimeter at 32.00 and 37.00 °C, respectively. On the basis of the metabolic thermogenic curves, some important biothermokinetic parameters such as the microbial growth rate constant (k), inhibition ratio (I) and half inhibition concentration (IC50) were estimated. The experimental results indicated that both the Schiff base ligand and its bismuth(III) complex could inhibit the growth metabolism of S. pombe and H. pylori, but the inhibitory abilities of the two compounds were as follows: the Schiff base ligand > the complex for S. pombe and the Schiff base ligand < the complex for H. pylori, respectively. More importantly, the results also found that the Schiff base bismuth(III) complex exhibited bidirectional biological effect and hormesis effect on H. pylori cell line, i.e., the complex can stimulate the growth metabolism of H. pylori at low concentration, while inhibiting its growth at high concentration.







Similar content being viewed by others
References
Wang Z. Chemical dictionary. Beijing: Chemical Industry Press (CIP); 2010. p. 445.
Li X, Jiang JH, Gu HW, Xiao SX, Li CH, Ye LJ, Li X, Li QG, Xu F, Sun LX. Calorimetric determination of the standard molar enthalpies of formation of o-vanillin and trimethoprim. J Therm Anal Calorim. 2015;119:721–6.
Li X, Jiang JH, Xiao SX, Gu HW, Li CH, Ye LJ, Li X, He DG, Yao FH, Li QG. Synthesis, thermodynamic properties and BSA interaction of a new Valen Shiff base derived from o-vanillin and trimethoprim. Thermochim Acta. 2014;575:291–9.
Xie JQ, Li CH, Dong JX, Qu W, Pan L, Peng ML, Xie MA, Tao X, Yu CM, Zhu Y, Zhang PH, Tang CG, Li QG. The standard molar enthalpy of formation of a new copper(II) Schiff-base complex and its interaction with bovine serum albumin. Thermochim Acta. 2014;598:7–15.
Li X, Jiang JH, Han BX, Gu HW, Xie ZF, Chen L, Xiao SX, Li CH, Li AT, Li X, Yao FH, Wang Q, Li QG. Synthesis and biological activities of o-vanillin-histidine Schiff-base and lanthanum Schiff-base complex. Chem J Chin Univ. 2015;36:856–63.
Li CH, Jiang JH, Li X, Tao LM, Xiao SX, Gu HW, Zhang H, Jiang C, Xie JQ, Peng MN, Pan LL, Xia XM, Li QG. Synthesis, crystal structure and biological properties of a bismuth(III) Schiff-base complex. RSC Adv. 2015;5:94267–75.
Galić N, Cimerman Z, Tomišić V. Spectrometric study of tautomeric and protonation equilibria of o-vanillin Schiff base derivatives and their complexes with Cu(II). Spectrochim Acta, Part A. 2008;71:1274–80.
Li X, Li CH, Jiang JH, Gu HW, Wei DL, Ye LJ, Hu JL, Xiao SX, Guo DC, Li X, Zhang H, Li QG. Synthesis and microcalorimetric determination of the bioactivities of a new Schiff base and its bismuth(III) complex derived from o-vanillin and 2,6-pyridinediamine. J Therm Anal Calorim. 2017;127:1767–76.
Phipps MA, Mackin LA. Application of isothermal microcalorimetry in solid state drug development. Pharm Sci Technol Today. 2000;3:9–17.
Li MX, Yang M, Niu J, Zhang LZ, Xie SQ. A nine-coordinated bismuth(III) complex derived from pentadentate 2,6-diacetylpyridine bis(4 N-methylthiosemicarbazone): crystal structure and both in vitro and in vivo biological evaluation. Inorg Chem. 2012;51:12521–6.
Backes GL, Neumann DM, Jursic BS. Synthesis and antifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides. Bioorg Med Chem. 2014;22:4629–36.
Friedman M, Henika PR, Mandrell RE. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2003;66:1811–21.
Jeewoth T, Bhowon MG, Wah HLK. Synthesis, characterization and antibacterial properties of Schiff bases and Schiff base metal complexes derived from 2,3-diaminopyridine. Transit Metal Chem. 1999;24:445–8.
Li X, Jiang JH, Chen QQ, Xiao SX, Li CH, Gu HW, Zhang H, Hu JL, Yao FH, Li QG. Synthesis of nordihydroguaiaretic acid derivatives and their bioactivities on S. pombe and K562 cell lines. Eur J Med Chem. 2013;62:605–13.
Zhou YM, Ye XR, Xin FB, Xin XQ. Solid state self-assembly synthesis of cobalt(II), nickel(II), copper(II) and zinc(II) complexes with a bis-Schiff base. Transit Metal Chem. 1999;24:118–20.
Ilhan S, Temel H, Yilmaz I, Şekerci M. Synthesis and characterization of new macrocyclic Schiff base derived from 2,6-diaminopyridine and 1,7-bis(2-formylphenyl)-1,4,7 trioxaheptane and its Cu(II), Ni(II), Pb(II), Co(II) and La(III) complexes. Polyhedron. 2007;26:2795–802.
Kaya İ, Bilici A, Gül M. Schiff base substitute polyphenol and its metal complexes derived from o-vanillin with 2,3-diaminopyridine: synthesis, characterization, thermal, and conductivity properties. Polym Adv Technol. 2008;19:1154–63.
Acknowledgements
The authors would like to acknowledge the financial supports from the National Natural Science Foundation of China (Grant Nos. 21273190 and 20973145). Prof. Qiang-Guo Li is the chief-director of these fund projects.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors claim that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Jiang, JH., Gu, HW. et al. Synthesis and biothermokinetic study of a new Schiff base and its bismuth(III) complex on the growth metabolism of S. pombe and H. pylori cell lines. J Therm Anal Calorim 132, 1913–1922 (2018). https://doi.org/10.1007/s10973-018-7101-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7101-6