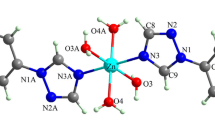
Abstract
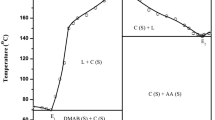
The solid–liquid equilibrium phase diagram and thermochemical studies on two binary organic systems involving N-methylurea (MU) with 4-nitrophenol (NP) and 3-nitrobenzoic acid NBA were studied. Both systems show the formation of an equimolar intermolecular compound (IMC) and two eutectics (E1 and E2) one on either side of the IMC. Thermodynamic parameters such as heat of mixing, entropy of fusion, roughness parameter, interfacial energy and excess thermodynamic functions of intermolecular compounds and eutectics were calculated using the experimentally determined enthalpy of fusion by the DSC method. The spectroscopic studies, FTIR and NMR, of both the IMCs along with their parent compounds revealed the hydrogen bonding association between the parent moieties forming the complex. The findings were also confirmed by the appearance of new peaks in the powder XRD of the complexes in addition to their parent compounds.









Similar content being viewed by others
References
Cave GWV, Raston CL, Scott JL. Recent advances in solventless organic reactions: towards benign synthesis with remarkable versatility. Chem Commun. 2001;21:2159–69.
Anastas PT, Warner JC. Green chemistry: theory and practice. New York: Oxford University Press; 1998.
Clark JH. Green chemistry: challenges and opportunities. Green Chem. 1999;1(1):1–8.
Garay AL, Pichon A, James SL. Solvent-free synthesis of metal complexes. Chem Soc Rev. 2007;36(6):846–55.
Houton KA, Burslem GM, Wilson AJ. Development of solvent-free synthesis of hydrogen-bonded supramolecular polyurethanes. Chem Sci. 2015;6(4):2382–8.
Tanaka K, Toda F. Solvent-free organic synthesis. Chem Rev. 2000;100(3):1025–74.
Desiraju GR. A bond by any other name. Angew Chem Int Ed. 2011;50(1):52–9.
Rai US, Singh M, Rai RN. Remarkable dielectric properties of 1: 2 inter-molecular compound of 2-(4-(dimethylamino)benzylideneamino) benzoic acid and urea due to excited-state intramolecular proton transfer. RSC Adv. 2017;7(55):34382–91.
Rai RN, Ramasamy P, Lan CW. Synthesis and crystal growth of binary organic NLO material UNBA. J Cryst Growth. 2002;235(1):499–504.
Singh NB, Glicksman ME, Mazelsky R. Solidification behaviour of organic nonlinear optical crystals. Prog Cryst Growth Charact Mater. 1988;17(4):265–78.
Nandi N, Bhattacharyya K, Bagchi B. Dielectric relaxation and solvation dynamics of water in complex chemical and biological systems. Chem Rev. 2000;100(6):2013–46.
Chemla DS, Zyss J. Nonlinear optical properties of organic molecules and crystals academic. New York: Elsevier; 1987.
Maeda M. Laser dyes. New York: Academic Press; 1984.
Winnik FM, Regismond ST. Fluorescence methods in the study of the interactions of surfactants with polymers. Colloids Surf A. 1996;118(1):1–39.
Saroja G, et al. The fluorescence response of a structurally modified 4-aminophthalimide derivative covalently attached to a fatty acid in homogeneous and micellar environments. J Phys Chem B. 1999;103(15):2906–11.
Singh NB, et al. Nonlinear optical characteristics of binary organic systems. J Cryst Growth. 1993;128:976–80.
Dwivedi Y, et al. Synthesis, physicochemical and optical characterization of novel fluorescing complex: o-phenylenediamine–benzoin. J Fluoresc. 2011;21(3):1255–63.
Henningsen T, et al. Growth of binary organic NLO crystals: mNa-pNA and mNa-CNA systems. Mater Lett. 1994;20(3-4):203–9.
Huiszoon C, Tiemessen GWM. Monomethylurea: a redetermination. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem. 1976;32(5):1604–6.
Wojcik G, Mossakowska I. Polymorphs of p-nitrophenol as studied by variable-temperature X-ray diffraction and calorimetry: comparison with m-nitrophenol. Acta Crystallogr B. 2006;62(1):143–52.
Dhaneshwar NN, et al. The crystal structure of a second modification of m-nitrobenzoic acid. Acta Crystallogr Sect B: Struct Crystallogr Cryst Chem. 1975;31(7):1978–80.
Dean JA. Lange’s handbook of chemistry. New York: McGraw-Hill; 1985.
Rai US, Singh M, Rai RN. Some physicochemical studies on organic eutectics and inter-molecular compounds. J Therm Anal Calorim. 2017;130(2):967–72.
Rai US, Rai RN. Physical chemistry of organic eutectic and monotectic: hexamethylbenzene–succinonitrile system. Chem Mater. 1999;11(11):3031–6.
Neupane U, Rai RN. Synthesis, spectral characterization, thermal and optical studies of novel complexes: 4-(dimethylamino)benzylidene- 4-acetamideaniline and 4-(dimethylamino)benzylidene-4-nitroaniline. J Fluoresc. 2017;27:2263–77.
Rai US, Singh M, Rai RN. Solid state synthesis, structural, physicochemical and optical properties of an inter-molecular compound: 2-hydroxy-1, 2-diphenylethanone-4-nitro-o-phenylenediamine system. J Solid State Chem. 2017;253:63–72.
Singh M, et al. Solid–liquid equilibrium, thermal, and physicochemical studies on salicylamide–4-nitrophenol and 2-cyanoacetamide–4-aminoacetophenone organic eutectic systems. J Therm Anal Calorim. 2013;113(2):977–83.
Calvaruso G, Ruggirello A, Turco V. Liveri. FT-IR investigation of the N-methylurea state in AOT reversed micelles. J Nanopart Res. 2002;4(3):239–46.
Abkowicz-Bieńko AJ, et al. Theoretical infrared spectrum and revised assignment for para-nitrophenol. Density functional theory studies. Chem Phys. 1999;250(2):123–9.
Samsonowicz M, et al. Experimental and theoretical IR, Raman, NMR spectra of 2-, 3-, and 4-nitrobenzoic acids. Int J Quantum Chem. 2007;107(2):480–94.
Etter MC, et al. Hydrogen bond-directed co-crystallization and molecular recognition properties of diaryl ureas. J Am Chem Soc. 1990;112(23):8415–26.
Ma G, et al. Thermal properties and reliability of eutectic mixture of stearic acid-acetamide as phase change material for latent heat storage. J Chem Thermodyn. 2017;106:178–86.
Qazi SJS, Rennie AR, Cockcroft JK, et al. Use of wide-angle X-ray diffraction to measure shape and size of dispersed colloidal particles. J Colloid Interface Sci. 2009;338:105–10.
Singh M, et al. Synthesis, crystal growth and physicochemical studies on a novel organic inter-molecular compound; 3, 5-dinitrobenzoic acid and salicylamide system. J. Crystal Growth. 2015;419:114–22.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Neupane, U., Rai, U.S. & Rai, R.N. Thermal, physicochemical and spectroscopic studies on some novel organic complexes obtained by green synthesis. J Therm Anal Calorim 132, 1741–1752 (2018). https://doi.org/10.1007/s10973-018-7051-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7051-z