Abstract
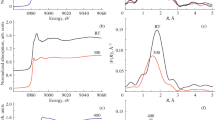
Copper-exchanged mordenites prepared by different methods, conventional and microwave assisted, have been investigated by TG, DSC, temperature-programmed reduction (TPR), and EPR methods. TPR study of hydrated and annealed at 573 and 973 K samples revealed that the copper reduction occurs directly from Cu2+ to Cu0, and Cu2+ ions are in different local environments. The microwave-assisted treatment leads to formation of [Cu–O–Cu]2+ species in the main mordenite channels. The most easily recoverable copper in the fully hydrated samples corresponds to the individual Cu2+ ions coordinated by H2O molecules, and the number of these [Cu(H2O)n]2+ complexes does not depend on the preparation method and the total copper content. According to the EPR study, upon sample dehydration, [Cu(H2O)n]2+ species lose water and approach the wall of the zeolite forming a bond with reversible charge transfer from a framework oxygen to Cu2+.




Similar content being viewed by others
References
Roy S, Hegde MS, Madras G. Catalysis for NOx abatement. Appl Energy. 2009;86:2283–97.
Chen Y, Cheng D, Chen F, Zhan X. NO decomposition and selective catalytic reduction of NO over Cu-ZSM-5 zeolite. Prog Chem. 2014;26:248–58.
Jabłońska M, Palkovits R. Copper based catalysts for the selective ammonia oxidation into nitrogen and water vapour—recent trends and open challenges. Appl Catal B Environ. 2016;181:332–51.
Yahiro H, Iwamoto M. Copper ion-exchanged zeolite catalysts in deNOx reaction. Appl Catal A. 2001;222:163–81.
Ali IO, El Sayed HA, El-Nasser KS, Shabana AA. Improvement in thermal stability and catalytic activity of titanium containing mordenite by cerium using hydrothermal methods for hydroxylation of benzene into phenol. J Therm Anal Calorim. 2016;123:1129–39.
Roushan AH, Omrani A, Kavian S, Azizi SN. Determination of degradation kinetics of polyvinyl alcohol/X-zeolite nanocomposite. J Therm Anal Calorim. 2017;128:1057–66.
Chassaing S, Beneteau V, Louis B, Pale P. Zeolites as green catalysts for organic synthesis: the cases of H-, Cu- & Sc-zeolites. Curr Org Chem. 2017;21:779–93.
Lamberti C, Zecchina A, Groppo E, Bordiga S. Probing the surfaces of heterogeneous catalysts by in situ IR spectroscopy. Chem Soc Rev. 2010;39:4951–5001.
Borkow G, Gabbay J. Copper as a biocidal tool. Curr Med Chem. 2005;12:2163–75.
Longano D, Ditaranto N, Sabbatini L, Torsi L, Cioffi N. Synthesis and antimicrobial activity of copper nanomaterials. In: Cioffi N, Rai M, editors. Nano-antimicrobials. Berlin: Springer; 2011. p. 85–117.
Drelich J, Li BW, Villeneuve B, Bowen P. Inexpensive mineral copper materials with antibacterial surfaces. Surf Innov. 2012;1:15–26.
Petranovskii V, Panina L, Bogomolova E, Belostotskaya G. Microbiologically active nanocomposite media. Proc SPIE Complex Med IV Beyond Linear Isotropic Dielectr. 2003;5218:244–55.
Blel W, Limousy L, Dutournié P, Ponche A, Boucher A, Le Fellic M. Study of the antimicrobial and antifouling properties of different oxide surfaces. Environ Sci Pollut Res. 2017;24:9847–58.
Jacob J, Chia LHL, Boey FYC. Thermal and non-thermal interaction of microwave radiation with materials. J Mater Sci. 1995;30:5321–7.
Zhukov YM, Efimov AY, Shelyapina MG, Petranovskii V, Zhizhin EV, Burovikhina A, et al. Effect of preparation method on the valence state and encirclement of copper exchange ions in mordenites. Microporous Mesoporous Mater. 2016;224:415–9.
Zhukov YM, Shelyapina MG, Zvereva IA, Efimov AY, Petranovskii V. Microwave assisted versus convention Cu2+ exchange in mordenite. Microporous Mesoporous Mater. 2018;259:220–8.
Utkina T, Chislov M, Silyukov O, Burovikhina A, Alexeevna Zvereva I. TG and DSC investigation of water intercalation and protonation processes in perovskite-like layered structure of titanate K2Nd2Ti3O10. J Therm Anal Calorim. 2016;125:281–7.
Vieira Dos Santos BR, Montoya Urbina M, Souza MJB, Garrido Pedrosa AM, Silva AOS, Sobrinho EV, et al. Preparation and characterization of Pt-dealuminated y zeolite by TG/DTA and TPR. J Therm Anal Calorim. 2015;119:391–9.
Ye Q, Huang Z, Hao Y, Wang J, Yang X, Fan X. Kinetic study of thermal degradation of poly(l-lactide) filled with β-zeolite. J Therm Anal Calorim. 2016;124:1471–84.
Krylova EA, Shelyapina MG, Nowak P, Harańczyk H, Chislov M, Zvereva IA, et al. Mobility of water molecules in sodium- and copper-exchanged mordenites: thermal analysis and 1H NMR. Microporous Mesoporous Mater. 2018;to be published.
Vanelderen P, Snyder BER, Tsai M-L, Hadt RG, Vancauwenbergh J, Coussens O, et al. Spectroscopic definition of the copper active sites in Mordenite: selective methane oxidation. J Am Chem Soc. 2015;137:6383–92.
Grundner S, Markovits MAC, Li G, Tromp M, Pidko EA, Hensen EJM, et al. Single-site trinuclear copper oxygen clusters in mordenite for selective conversion of methane to methanol. Nat Commun. 2015;6:7546-1–9.
Abragam A, Bleaney B. Electron paramagnetic resonance of transition ions. Oxford: Oxford University Press; 1970.
Carl PJ, Larsen SC. EPR study of copper-exchanged zeolites: effects of correlated g- and A-strain, Si/Al ratio, and parent zeolite. J Phys Chem B. 2000;104:6568–75.
Kucherov AV, Slinkin AA, Kondrat’ev DA, Bondarenko TN, Rubinstein AM, Minachev KM. Cu2+—cation location and reactivity in mordenite and ZSM-5e.s.r.-study. Zeolites. 1985;5:320–4.
Petranovskii V, Gurin V, Bogdanchikova N, Licea A, Sugi Y, Stoyanov E. The effect of SiO2/Al2O3 molar ratio in mordenite upon the optical appearance of reduced copper. Mater Sci Eng A. 2002;332:174–83.
Zhukov YM, Efimov AY, Petranovskii V, Zhizhin EV, Pudikov DA. Effect of the exposure time at recording an X-ray photoelectron spectrum on the charge state and environment of copper in mordenites. JETP Lett. 2016;103:399–402.
Schoonheydt RA. Transition metal ions in zeolites: siting and energetics of Cu2+. Catal Rev Sci Eng. 1993;35:129–68.
Delabie A, Pierloot K, Groothaert MH, Weckhuysen BM, Schoonheydt RA. The siting of Cu(II) in mordenite: a theoretical spectroscopic study. Phys Chem Chem Phys. 2002;4:134–45.
Oliva C, Selli E, Ponti A, Correale L, Solinas V, Rombi E, et al. FTIR and EPR characterisation of copper-exchanged mordenites and beta zeolites. J Chem Soc Faraday Trans. 1997;93:2603–8.
Modén B, Da Costa P, Lee DK, Iglesia E. Transient studies of oxygen removal pathways and catalytic redox cycles during NO decomposition on Cu–ZSM5. J Phys Chem B. 2002;106:9633–41.
Da Costa P, Modén B, Meitzner GD, Lee DK, Iglesia E. Spectroscopic and chemical characterization of active and inactive Cu species in NO decomposition catalysts based on Cu-ZSM5. Phys Chem Chem Phys. 2002;4:4590–601.
Lee DK. Quantification and redox property of the oxygen-bridged Cu2+ dimers as the active sites for the NO decomposition over Cu-ZSM-5 catalysts. Korean J Chem Eng. 2004;21:611–20.
Narsimhan K, Iyoki K, Dinh K, Román-Leshkov Y. Catalytic oxidation of methane into methanol over copper-exchanged zeolites with oxygen at low temperature. ACS Cent Sci. 2016;2:424–9.
Alayon EMC, Nachtegaal M, Bodi A, Van Bokhoven JA. Reaction conditions of methane-to-methanol conversion affect the structure of active copper sites. ACS Catal. 2014;4:16–22.
Bozbag SE, Alayon EMC, Pecháček J, Nachtegaal M, Ranocchiari M, van Bokhoven JA. Methane to methanol over copper mordenite: yield improvement through multiple cycles and different synthesis techniques. Catal Sci Technol. 2016;6:5011–22.
Acknowledgements
The studies were carried out at the Research Park of Saint Petersburg State University: Centre of Thermal Analysis and Calorimetry and Centre for Magnetic Resonance. This work was partially supported by DGAPA-UNAM IN107817 Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shelyapina, M., Zvereva, I., Yafarova, L. et al. Thermal analysis and EPR study of copper species in mordenites prepared by conventional and microwave-assisted methods. J Therm Anal Calorim 134, 71–79 (2018). https://doi.org/10.1007/s10973-018-7016-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7016-2