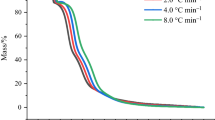
Abstract
Azo compounds are usually used as initiators and blowing agents. They are also typically self-reactive materials capable of undergoing a runaway reaction during storage or transportation, which can cause serious fires and explosions. To prevent the thermal hazard of azos occurring in a real process, transportation, or storage, azo initiator 2,2′-azobis (2-methylpropionamide) dihydrochloride (AIBA), which has few studies on relevant research in thermal safety, was selected to be investigated. First, the features of thermal decomposition under non-isothermal condition of AIBA were attained through differential scanning calorimetry and simultaneous thermal analysis. Second, the collected data were substituted into mathematical analyzer to evaluate the basic thermal hazard for AIBA. In addition, based on Semenov theoretical model and thermokinetic parameters, the critical ignition temperature (TCI) was extrapolated for consideration of surroundings temperature under specific cooling system (TS). The results provided process control data and the consequences of thermal runaway for AIBA. In addition, the related numerical methods for prevention of thermal runaway reaction could be calculated during process deviation. Therefore, the assessment conclusions also showed that process parameters must be measured and controlled strictly to generate the desired reaction. The results showed that the various TCI were all less than 70 °C. Therefore, it is essential to avoid a temperature beyond the TCI or cooling system failure.











Similar content being viewed by others
Abbreviations
- R :
-
Gas constant/8.31415 J K−1 mol−1
- A :
-
Pre-exponential factor of Arrhenius equation (min−1)
- A(α):
-
Pre-exponential factor at conversion (min−1)
- A′(α):
-
Amended pre-exponential factor by a product of \(A (\alpha )\) and \(f (\alpha )\) (min−1)
- C o :
-
Original concentration of the material (g cm−3)
- C :
-
Concentration of the material (g cm−3)
- C p :
-
Specific heat of material (J g−1 K−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- E(α):
-
Apparent activation energy factor at conversion (kJ mol−1)
- h :
-
Heat exchange capability index of the cooling system (kJ m−2 K−1 min−1)
- k 0 :
-
Reaction rate constant (s−1)
- n :
-
Reaction order (dimensionless)
- m :
-
Mass of material (g)
- ΔH d :
-
Heat of decomposition (J g−1)
- ΔH t :
-
Heat of decomposition at time (J g−1)
- ΔH total :
-
Total heat of decomposition (J g−1)
- r :
-
Reaction rate (s−1)
- q g :
-
Heat production rate (kJ min−1)
- q r :
-
Heat removal rate (kJ min−1)
- q r1 :
-
Heat removal rate by high cooling medium (kJ min−1)
- q r2 :
-
Heat removal rate by cooling system (kJ min−1)
- q r3 :
-
Heat removal rate by low cooling system (kJ min−1)
- S :
-
Effective heat exchange area (m2)
- V :
-
Volume of process instruments (m3)
- T :
-
Process temperature (K)
- T 0 :
-
Apparent exothermic temperature (K)
- T P :
-
Temperature at the maximum heat release in reaction (K)
- T S :
-
Surrounding temperature under cooling system (K)
- t :
-
Reaction time (min)
- T CI :
-
Critical ignition or extinction temperature (K)
- T CE :
-
Critical extinguishing temperature (K)
- T SE :
-
Stable point of extinguishing temperature (K)
- TSI :
-
Stable point of ignition temperature (K)
- T SL :
-
Stable point at lower temperature (K)
- T SH :
-
Stable point at higher temperature (K)
- T M :
-
Cutoff point between curves qg and qr at the highest and lowest cooling efficient system (K)
- X A :
-
Fractional conversion (dimensionless)
- α :
-
Degree of conversion (dimensionless)
References
United Nations. Recommendations on the transport of dangerous goods: model regulations. 12th Revised ed. New York: United Nations Publications; 2018.
Liu SH, Cheng YF, Meng XR, Ma HH, Song SX, Liu WJ, Shen ZW. Influence of particle size polydispersity on coal dust explosibility. J Loss Prev Process Ind. 2018;56:444–50.
Dubikhin V, Knerel’man E, Manelis G, Nazin G, Prokudin V, Stashina G, Chukanov N, Shastin A. Thermal decomposition of azobis (isobutyronitrile) in the solid state. Cage effect. Recombination and disproportionation of cyanoisopropyl radicals. Dokl Phys Chem. 2012;446(2):171–5.
Gowda S, Abiraj K, Gowda DC. Reductive cleavage of azo compounds catalyzed by commercial zinc dust using ammonium formate or formic acid. Tetrahedron Lett. 2002;43(7):1329–31.
Liu SH, Yu YP, Lin YC, Weng SY, Hsieh TF, Hou HY. Complex thermal evaluation for 2,2′-azobis(isobutyronitrile) by non-isothermal and isothermal thermokinetic analysis methods. J Therm Anal Calorim. 2014;116(3):1361–7.
Kossoy AA, Belokhvostov VM, Koludarova EY. Thermal decomposition of AIBN: Part D: verification of simulation method for SADT determination based on AIBN benchmark. Thermochim Acta. 2015;621:36–43.
Liu SH, Shu CM. Advanced technology of thermal decomposition for AMBN and ABVN by DSC and VSP2. J Therm Anal Calorim. 2015;121(1):533–40.
Liu SH, Lin WC, Hou HY, Shu CM. Comprehensive runaway thermokinetic analysis and validation of three azo compounds using calorimetric approach and simulation. J Loss Prev Process Ind. 2017;49:970–82.
Berdouzi F, Villemur C, Olivier Maget N, Gabas N. Dynamic simulation for risk analysis: application to an exothermic reaction. Process Saf Environ Prot. 2018;113:149–63.
Wu KW, Hou HY, Shu CM. Thermal phenomena studies for dicumyl peroxide at various concentrations by DSC. J Therm Anal Calorim. 2006;83(1):41–4.
Shen SJ, Wu SH, Chi JH, Wang YW, Shu CM. Thermal explosion simulation and incompatible reaction of dicumyl peroxide by calorimetric technique. J Therm Anal Calorim. 2010;102(2):569–77.
Zhu Y, Chen Y, Zhang L, Li W, Huang B, Wu J. Numerical investigation and dimensional analysis of reaction runaway evaluation for thermal polymerization. Chem Eng Res Des. 2015;104:32–41.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, Opfermann J, Strey R, Anderson HL, Kemmler A, Keuleers R, Janssens J, Desseyn HO, Li CR, Tang TB, Roduit B, Malek J, Mitsuhashi T. Computational aspects of thermokinetic analysis: Part A: the ICTAC thermokinetic project-data, methods and results. Thermochim Acta. 2000;355(1):125–43.
Kossoy AA, Benin A, Akhmetshin Y. An advanced approach to reactivity rating. J Hazard Mater. 2005;118(1):9–17.
Di Somma I, Andreozzi R, Canterino M, Caprio V, Sanchirico R. Thermal decomposition of cumene hydroperoxide: chemical and thermokinetic characterization. AlChE J. 2008;54(6):1579–84.
Chiang CL, Liu SH, Lin YC, Shu CM. Thermal release hazard for the decomposition of cumene hydroperoxide in the presence of incompatibles using differential scanning calorimetry, thermal activity monitor III, and thermal imaging camera. J Therm Anal Calorim. 2017;127(1):1061–9.
Wang SY, Kossoy AA, Yao YD, Chen LP, Chen WH. Kinetics-based simulation approach to evaluate thermal hazards of benzaldehyde oxime by DSC tests. Thermochim Acta. 2017;655:319–25.
Burnham AK. Computational aspects of thermokinetic analysis Part D: the ICTAC thermokinetic project—multi-thermal-history model-fitting methods and their relation to isoconversional methods. Thermochim Acta. 2000;355(1):165–70.
Maciejewski M. Computational aspects of thermokinetic analysis Part B: the ICTAC thermokinetic Project—the decomposition thermokinetic of calcium carbonate revisited, or some tips on survival in the thermokinetic minefield. Thermochim Acta. 2000;355(1):145–54.
Roduit B. Computational aspects of thermokinetic analysis Part E: the ICTAC Thermokinetic project—numerical techniques and thermokinetic of solid state processes. Thermochim Acta. 2000;355(1):171–80.
Vyazovkin S. Computational aspects of thermokinetic analysis Part C. The ICTAC thermokinetic project—the light at the end of the tunnel. Thermochim Acta. 2000;355(1):155–63.
Semenov NN. Thermal theory of combustion and explosion. Washington: National Advisory Committee for Aeronautics; 1942.
Talouba IB, Balland L, Mouhab N, Abdelghani-Idrissi M. Thermokinetic parameter estimation for decomposition of organic peroxides by means of DSC measurements. J Loss Prev Process Ind. 2011;24(4):391–6.
Liu SH, Lin WC, Xia H, Hou HY, Shu C-M. Combustion of 1-butylimidazolium nitrate via DSC, TG, VSP2, FTIR, and GC/MS: an approach for thermal hazard, property and prediction assessment. Process Saf Environ Prot. 2018;116:603–14.
Zhang B, Liu SH, Chi JH. Thermal hazard analysis and thermokinetic calculation of 1,3-dimethylimidazolium nitrate via TG and VSP2. J Therm Anal Calorim. 2018;134:2367–74.
Roduit B, Borgeat C, Berger B, Folly P, Andres H, Schädeli U, Vogelsanger B. Up-scaling of DSC data of high energetic materials: simulation of cook-off experiments. J Therm Anal Calorim. 2006;85(1):195–202.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6.
Ozawa T. Thermal analysis—review and prospect. Thermochim Acta. 2000;355(1–2):35–42.
ASTM. Standard test method for Arrhenius thermokinetic constants for thermally unstable materials. Philadelphia: American Society for Testing and Materials; 1979.
Boswell P. On the calculation of activation energies using a modified Kissinger method. J Therm Anal Calorim. 1980;18(2):353–8.
Kossoy AA, Hofelich T. Methodology and software for assessing reactivity ratings of chemical systems. Process Saf Prog. 2003;22(4):235–40.
Kossoy AA, Sheinman IY. Evaluating thermal explosion hazard by using thermokinetic-based simulation approach. Process Saf Environ Prot. 2004;82(6):421–30.
Huang J, Jiang J, Ni L, Zhang W, Shen S, Zou M. Thermal decomposition analysis of 2,2-di-(tert-butylperoxy)butane in non-isothermal condition by DSC and GC/MS. Thermochim Acta 2018. https://www.sciencedirect.com/science/article/pii/S0040603118302326.
Filimonov VY, Koshelev KB, Sytnikov AA. Thermal modes of heterogeneous exothermic reactions. Solid-phase interaction. Combust Flame. 2017;185:93–104.
Semenov NN. Zur theorie des verbrennungsprozesses. Zeitschrift für Physik. 1928;48(7–8):571–82.
Lu G, Zhang C, Chen L, Chen W, Yang T, Zhou Y. Kinetic analysis and self-accelerating decomposition temperature (SADT) of β-nitroso-α-naphthol. Process Saf Environ Prot. 2015;95:69–76.
Acknowledgements
The authors wish to express their gratitude to Dr. Arcady A. Kossoy of ChemInform Saint Petersburg, Federation, Russian, for providing technical assistance. The authors would also like to thank Dr. Shang-Hao Liu and Dr. Kuo-Ming Luo for their help on the measurements of critical parameters.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, CR., Shu, CM. Kinetic modeling for thermal hazard of 2,2′-azobis (2-methylpropionamide) dihydrochloride using calorimetric approach and simulation. J Therm Anal Calorim 137, 1021–1030 (2019). https://doi.org/10.1007/s10973-018-07995-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-07995-8