Abstract
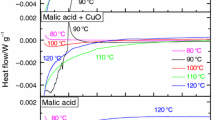
Cosmeceutical products that contain malic acid (MA), salicylic acid (SA), and hyaluronic acid as well as a variety of antioxidants are used worldwide. Therefore, safer ingredients of cosmeceutical products have become an important issue based on sales volume. In general, the chemical composition may affect the thermal stability of a cosmeceutical product. Temperature changes may occur in the manufacturing, storage, and transport of the product, affecting its stability. Because cosmeceutical products are placed directly on the skin, sensitivity has become an increasing concern. However, potential risks have not been clearly identified. To investigate the thermal stability behavior of regular cosmeceutical materials, thermogravimetry and differential scanning calorimetry have been used. For this study, the thermal stability of MA and SA was studied, and the acids were individually mixed with CuO or Fe2O3 to evaluate the effect of adding metal oxides. According to the DSC curves, the apparent exothermic onset temperature occurred when MA and SA were mixed with Fe2O3. Apparent activation energy values of individual samples calculated using the ASTM E698 and Ozawa–Flynn–Wall methods ranged from 72.2 to 87.4 kJ mol−1 and from 84.2 to 98.7 kJ mol−1, respectively. The results can be used to calculate the optimal parameters for safe cosmeceutical manufacturing and establishing a database of MA and SA for loss prevention protocols.
















Similar content being viewed by others
Abbreviations
- A :
-
Frequency factor (s−1)
- D :
-
Correction coefficient for apparent activation energy (dimensionless)
- E a :
-
Apparent activation energy (kJ mol−1)
- K :
-
Reaction rate constant (min−1)
- R :
-
Gas constant (8.314 J mol−1 K)
- T 0 :
-
Apparent exothermic onset temperature (°C)
- T p :
-
Peak temperature (°C)
- α :
-
Conversion degree (dimensionless)
- β :
-
Heating rate (°C min−1)
- ∆H d :
-
Heat of decomposition (J g−1)
References
Telegdi J, Trif L, Nagy E, Mihály J, Molnár N. New comonomers in malic acid polyesters. J Therm Anal Calorim. 2017;129(2):991–1000.
Van Staden J, Volschenk H, Van Vuuren H, Viljoen-Bloom M. Malic acid distribution and degradation in grape must during skin contact: the influence of recombinant malo-ethanolic wine yeast strains. S Afr J Enol Vitic. 2017;26(1):16–20.
Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial–ethnic groups. Dermatol Surg. 1999;25(1):18–22.
John J, Devi Rugmini S, Sreedharan Nair B. Kinetic analysis of thermal and hydrolytic decomposition of spiroborate ester of curcumin with salicylic acid. Orient J Chem. 2017;33(2):849–58.
Zhou ZZ, Chan HM, Sung HH, Tong HH, Zheng Y. Identification of new cocrystal systems with stoichiometric diversity of salicylic acid using thermal methods. Pharm Res. 2016;33(4):1030–9.
do Nascimento ALCS, Teixeira JA, Nunes WDG, Gomes DJC, Gaglieri C, Treu-Filho O, Pivatto M, Caires FJ, Ionashiro M. Thermal behavior of glycolic acid, sodium glycolate and its compounds with some bivalent transition metal ions in the solid state. J Therm Anal Calorim (in press).
Tsai YT, You ML, Qian XM, Shu CM. Calorimetric techniques combined with various thermokinetic models to evaluate incompatible hazard of tert-butyl peroxy-2-ethyl hexanoate mixed with metal ions. Ind Eng Chem Res. 2013;52(24):8206–15.
Deng J, Zhao JY, Huang AC, Zhang YN, Wang CP, Shu CM. Thermal behavior and microcharacterization analysis of second-oxidized coal. J Therm Anal Calorim. 2017;127(1):439–48.
Bisinella RZ, Ribeiro JC, de Oliveira CS, Colman TA, Schnitzler E, Masson ML. Some instrumental methods applied in food chemistry to characterise lactulose and lactobionic acid. Food Chem. 2017;220:295–8.
Bezerra GSN, Pereira MAV, Ostrosky EA, Barbosa EG, de Moura MdFV, Ferrari M, Aragão CFS, Gomes APB. Compatibility study between ferulic acid and excipients used in cosmetic formulations by TG/DTG, DSC and FTIR. J Therm Anal Calorim. 2016;127(2):1683–91.
Pardauil JJR, de Molfetta FA, Braga M, de Souza LKC, Filho GNR, Zamian JR, da Costa CEF. Characterization, thermal properties and phase transitions of amazonian vegetable oils. J Therm Anal Calorim. 2016;127(2):1221–9.
Deng J, Zhao JY, Xiao Y, Zhang YN, Huang AC, Shu CM. Thermal analysis of the pyrolysis and oxidation behaviour of 1/3 coking coal. J Therm Anal Calorim (in press).
Tsai YT, Lin SY, Tong JW, Chen WC, Chen WT, Shu CM. Incompatible hazard investigation of a cycloaliphatic epoxy resin using green analytical method. J Therm Anal Calorim. 2015;122(3):1135–41.
Tong JW, Chen WC, Tsai YT, Cao Y, Chen JR, Shu CM. Incompatible reaction for (3-4-epoxycyclohexane) methyl-3′-4′-epoxycyclohexyl-carboxylate (EEC) by calorimetric technology and theoretical kinetic model. J Therm Anal Calorim. 2014;116(3):1445–52.
Wang CP, Yang Y, Tsai YT, Deng J, Shu CM. Spontaneous combustion in six types of coal by using the simultaneous thermal analysis-Fourier transform infrared spectroscopy technique. J Therm Anal Calorim. 2016;126(3):1591–602.
Li KY, Tsai SY, Lin CP, Tsai YT, Shu CM. Smart technology for evaluating fire extinguishing effect of tert-butyl hydroperoxide. Ind Eng Chem Res. 2013;52(32):10969–76.
Huang AC, Chen WC, Huang CF, Zhao JY, Deng J, Shu CM. Thermal stability simulations of 1,1-bis(tert-butylperoxy)-3,3,5 trimethylcyclohexane mixed with metal ions. J Therm Anal Calorim. 2017;130(2):949–57.
Karl W, Perla R, Gérard C, Franck C, Luc NM, Hayat B, Denis F. Effect of surfactant on structure thermal behavior of cetyl stearyl alcohols. J Therm Anal Calorim. 2015;123(2):1411–7.
Kök M. Heating rate effect on the DSC kinetics of oil shales. J Therm Anal Calorim. 2007;90(3):817–21.
Tsai LC, Tsai YT, Lin CP, Liu SH, Wu TC, Shu CM. Isothermal versus non-isothermal calorimetric technique to evaluate thermokinetic parameters and thermal hazard of tert-butyl peroxy-2-ethyl hexanoate. J Therm Anal Calorim. 2012;109(3):1291–6.
Das M, Shu CM. A green approach towards adoption of chemical reaction model on 2,5-dimethyl-2,5-di-(tert-butylperoxy)hexane decomposition by differential isoconversional kinetic analysis. J Hazard Mater. 2016;301:222–32.
Chen WC, Shu CM. Prediction of thermal hazard for TBPTMH mixed with BPO through DSC and isoconversional kinetics analysis. J Therm Anal Calorim. 2016;126(3):1937–45.
Chen WC, Lin JR, Liao MS, Wang YW, Shu CM. Green approach to evaluating the thermal hazard reaction of peracetic acid through various kinetic methods. J Therm Anal Calorim. 2016;127(1):1019–26.
Liu SH, Cao CR, Lin YC, Shu CM. Using thermal analysis and kinetic calculation method to assess the thermal stability of 2,2′-azobis-(2-methylbutyronitrile). J Therm Anal Calorim (in press).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, AC., Chuang, YK., Huang, CF. et al. Thermokinetic analysis of the stability of malic and salicylic acids in cosmeceutical formulations containing metal oxides. J Therm Anal Calorim 132, 165–172 (2018). https://doi.org/10.1007/s10973-017-6870-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6870-7