Abstract
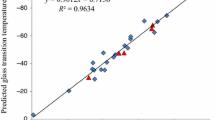
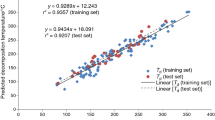
In this work, the most effective molecular descriptors on the thermal behaviour of energetic azido-ester plasticizers are investigated through quantitative structure–property relationship approach. At first, a new simple and reliable correlation is developed for predicting thermal decomposition temperature (T d) of energetic azido-ester plasticizers through multiple linear regression method. The determination coefficient of the derived correlation is 0.950, and it has root mean square deviation (RMSD) and average absolute deviation (AAD) of 2.74 and 2.09 °C, respectively. The internal and external validation method is shown that the developed correlation has good predictive ability. Then, the relationship between glass transition temperature (T G) of energetic azido-ester plasticizers and their T d is studied. This study shows that the optimum elemental composition, number of ester groups, number of azido groups and the presence of aromatic fragments are the important molecular descriptors as well as several non-additive structural parameters which have significant effect on the thermal behaviour of energetic azido-ester plasticizers. The determination coefficient of this correlation is 0.971, and it has RMSD and AAD of 3.70 and 2.67 °C, respectively. These results could be used to design ideal energetic azido-ester plasticizers.


Similar content being viewed by others
References
Ang HG, Pisharath S. Energetic polymers. Weinheim: Wiley; 2012. p. 171–9.
Kumari D, Balakshe R, Banerjee S, Singh H. Energetic plasticizers for gun & rocket propellants. Rev J Chem. 2012;2(3):240–62. https://doi.org/10.1134/S207997801203003X.
Provatas A. Energetic polymers and plasticizers for explosive formulations–a review of recent advances. DSTO. 2000.
Kumari D, Anjitha SG, Pant CS, Patil M, Singh H, Banerjee S. Synthetic approach to novel azido esters and their utility as energetic plasticizers. RSC Adv. 2014;4(75):39924–33. https://doi.org/10.1039/C4RA06530A.
Pant CS, Wagh RM, Nair JK, Gore GM, Venugopalan S. Synthesis and characterization of two potential energetic azido esters. Propell Explos Pyrot. 2006;31(6):477–81. https://doi.org/10.1002/prep.200600065.
Kumari D, Yamajala KDB, Singh H, Sanghavi RR, Asthana SN, Raju K, Banerjee s. Application of azido esters as energetic plasticizers for LOVA propellant formulations. Propell Explos Pyrot. 2013; 38(6): 805–9. http://doi.org/10.1002/prep.201300070.
Kumari D, Singh H, Patil M, Thiel W, Pant CS, Banerjee S. Synthesis, characterization, thermal and computational studies of novel tetra-azido esters as energetic plasticizer. Thermochim Acta. 2013;562:96–104. https://doi.org/10.1016/j.tca.2013.03.042.
Shaojun Q, Huiqing F. An azido ester plasticizer, 1,3-di(azidoacetoxy)-2,2-di(azidomethyl)propane (PEAA): synthesis, characterization and thermal properties. Propell Explos Pyrot. 2006;31(3):205–8. https://doi.org/10.1002/prep.200600028.
Pant CS, Wagh RM, Nair JK, Mukundan T, Venugopalan S. Synthesis and characterization of first generation dendritic azidoesters. Propell Explos Pyrot. 2007;32(6):461–7. https://doi.org/10.1002/prep.200700050.
Pant CS, Wagh RM, Nair JK, Mukundan T. Dendtritic azido ester: a potential energetic additive for high energy material (HEM) formulations. J Energ Mater. 2006;24(4):333–9. https://doi.org/10.1080/07370650600896681.
Unkelbach G, Keicher T, Krause H. Synthesis and characterization of new triazido-plasticizers. In: 36th international annual conference ICT. Karlsruhe, Germany. 2005; 49: 1–8.
Ghosh K, Pant CS, Sanghavin R, Adhav S, Singh A. Studies on triple base gun propellant based on two energetic azido esters. J Energ Mater. 2009;27(1):40–50. https://doi.org/10.1080/07370650802182542.
Agrawal JP, Bhongle RK, David FM, Nair JK. Bis(2-azido ethyl)adipate plasticizer: synthesis and characterization. J Energ Mater. 1993;11(1):67–83. https://doi.org/10.1080/07370659308018640.
Pourmortazavi SM, Rahimi-Nasrabadi M, Kohsari I, Hajimirsadeghi SS. Non-isothermal kinetic studies on thermal decomposition of energetic materials. J Therm Anal Calorim. 2012;110(2):857–63. https://doi.org/10.1007/s10973-011-1845-6.
Cusu JP, Musuc AM, Matache M, Oancea D. Kinetics of exothermal decomposition of some ketone-2,4-dinitrophenylhydrazones. J Therm Anal Calorim. 2012;110(3):1259–66. https://doi.org/10.1007/s10973-011-2040-5.
Lee JS, Hsu CK, Chang CL. A study on the thermal decomposition behaviors of PETN, RDX, HNS and HMX. Thermochim Acta. 2002;392–3:173–6. https://doi.org/10.1016/S0040-6031(02)00099-0.
Zhao-xu C, Heming X. Impact sensitivity and activation energy of pyrolysis for tetrazole compounds. Int J Quantum Chem. 2000;79(6):350–7. https://doi.org/10.1002/1097-461X(2000)79:6<350:AID-QUA3>3.0.CO;2-T.
Wypych G. Handbook of plasticizers. New York: ChemTec Publishing; 2004. p. 318.
Van krevelen DW, Nijenhuis KT. Properties of polymers. Amsterdam: Elsevier; 2009. p. 129–88.
Wunderlich B. Thermal analysis of polymeric materials. Netherlands: Springer; 2005. p. 79.
Zohari N, Keshavarz MH, Seyedsadjadi SA. A link between impact sensitivity of energetic compounds and their activation energies of thermal decomposition. J Therm Anal Calorim. 2014;117(1):423–32. https://doi.org/10.1007/s10973-014-3643-4.
Zohari N, Keshavarz MH, Seyedsadjadi SA. A novel method for risk assessment of electrostatic sensitivity of nitroaromatics through their activation energies of thermal decomposition. J Therm Anal Calorim. 2014;115(1):93–100. https://doi.org/10.1007/s10973-013-3328-4.
Keshavarz MH, Hayati M, Ghariban-Lavasani S, Zohari N. Relationship between activation energy of thermolysis and friction sensitivity of cyclic and acyclic nitramines. Z Anorg Allg Chem. 2016;642(2):182–8. https://doi.org/10.1002/zaac.201500706.
Keshavarz MH, Zohari N, Seyedsadjadi SA. Relationship between electric spark sensitivity and activation energy of the thermal decomposition of nitramines for safety measures in industrial processes. J Loss Prevent Proc. 2013;26(6):1452–6. https://doi.org/10.1016/j.jlp.2013.09.012.
Keshavarz MH, Zohari N, Seyedsadjadi SA. Validation of improved simple method for prediction of activation energy of the thermal decomposition of energetic compounds. J Therm Anal Calorim. 2013;114(2):497–510. https://doi.org/10.1007/s10973-013-3022-6.
Zohari N, Abrishami F, Sheibani N. A novel simple correlation for predicting glass transition temperature of energetic azido-ester plasticizers through molecular structures. J Therm Anal Calorim. 2017;127(3):2243–51. https://doi.org/10.1007/s10973-016-5738-6.
Zohari N, Keshavarz MH, Dalaei Z. Prediction of decomposition onset temperature and heat of decomposition of organic peroxides using simple approaches. J Therm Anal Calorim. 2016;125(2):887–96.
Keshavarz MH, Pouretedal HR, Saberi E. A new method for predicting decomposition temperature of imidazolium-based energetic ionic liquids. Z Anorg Allg Chem. 2017;643(2):171–9.
Keshavarz MH, Esmaeilpour K, Taghizadeh H. A new approach for assessment of glass transition temperature of acrylic and methacrylic polymers from structure of their monomers without using any computer codes. J Therm Anal Calorim. 2016;126(3):1787–96.
Gharagheizi F, Keshavarz MH, Ilani-Kashkouli P, Farahani N, Tumba K. A group contribution method for estimation of glass-transition temperature of 1, 3-dialkylimidazolium ionic liquids. J Therm Anal Calorim. 2013;114(3):1363–82.
Keshavarz MH, Esmaeilpour K, Saani MH, Taghizadeh H. A new method for assessment of glass transition temperature of ionic liquids from structure of their cations and anions without using any computer codes. J Therm Anal Calorim.:1–9.
Palm WJ III. Introduction to matlab for engineers. New York: McGraw-Hill; 2005. p. 4–328.
Agrawal JP, Hodgson RD. Organic chemistry of explosives. Chichester: Wiley; 2007. p. 333–9.
Bräse S, Banert K. Organic azides: syntheses and applications. Chippenham: Wiley; 2010. p. 53.
Hunter CA, Sanders JK. The nature of π–π interactions. J Am Chem Soc. 1990;112:5525–34.
Tropsha A. Best practices for QSAR model development, validation, and exploitation. Mol Inform. 2010;29(6–7):476–88. https://doi.org/10.1002/minf.201000061.
Gramatica P. Principles of QSAR models validation: internal and external. QSAR and Comb Sci. 2007;26(5):694–701. https://doi.org/10.1002/qsar.200610151.
Acknowledgement
We would like to thank the research committee of Malek-ashtar University of Technology (MUT) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zohari, N., Sheibani, N. & Chavoshi, H.Z. Investigation of the most effective molecular descriptors on the thermal behaviour of energetic azido-ester plasticizers through QSPR approach. J Therm Anal Calorim 131, 3157–3167 (2018). https://doi.org/10.1007/s10973-017-6809-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6809-z