Abstract
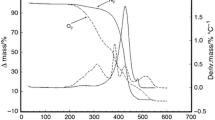
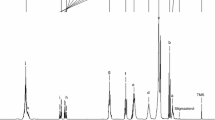
Sapucaia (Lecythis pisonis) is a tree that grows in Colombia, Venezuela and the Guyanas and is widely distributed in Brazil. This work presents a study of sapucaia nut oils (SO) that were obtained by Bligh and Dyer (LP1) and Soxhlet (LP2) methods and were evaluated for their fatty acid composition, rheological and thermal properties, total phenolic compounds (TPC), antioxidant properties and oxidative stability using Rancimat and ATR-FTIR spectroscopy. The analyses showed that the method of extraction impacts the fatty acid profiles of SO. Oil extracts present considerable TPC content and antioxidant properties. Thermal analysis revealed three degradation steps for SO in the air atmosphere, starting at around 130 °C, being thermally stable up to 300 °C (with a ~ 5% mass loss) and reaching total degradation near 620 °C. Thermal analysis under N2 produced two degradation steps, initiating at around 130 °C and finishing at 500 °C. Rancimat also confirmed the high thermal stability of SO, with induction periods of 13.28 h (LP1) and 7.18 h (LP2). The DSC parameters of SO were similar among each other. Crystallization (− 8.04 to − 73.93 °C) and melting (− 31.34 to 8.28 °C) phases occurred over a large temperature range. SO presented FTIR spectral features with characteristic bands for vegetable oils. Ostwald–de Waele and Herschel–Bulkley rheological models indicated major pseudoplastic behavior for SO, with a predominant viscous component. These results reinforce that SO are appropriate for human consumption and open up new possibilities for their industrial exploitation, such as for food and the cosmetic, pharmaceutical and biodiesel industries.









Similar content being viewed by others
Abbreviations
- SO:
-
Sapucaia nut oil (s)
- LP1:
-
Oil extracted by Bligh and Dyer
- LP2:
-
Oil extracted by Soxhlet
- TPC:
-
Total phenolic compounds
- Trolox:
-
6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
- TPTZ:
-
2,4,6-Tri(2-pyridyl)-s-triazine
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- ABTS:
-
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- FRAP:
-
Ferric reducing antioxidant power
- TE:
-
Trolox equivalent
- GA:
-
Gallic acid
- GAE:
-
Gallic acid equivalents
- OSI:
-
Oxidative stability index
- IP:
-
Induction period
- ATR-FTIR:
-
Attenuated total reflectance Fourier transform infrared spectroscopy
- FA:
-
Fatty acid
- SFA:
-
Saturated fatty acid
- UFA:
-
Unsaturated fatty acid
- MUFA:
-
Monounsaturated fatty acid
- PUFA:
-
Polyunsaturated fatty acid
- TAG:
-
Triacylglycerol
- SFC:
-
Solid fat content
- OW:
-
Ostwald–de Waele
- HB:
-
Herschel–Bulkley
References
Reuters T. Web of science. Web Sci. 2017 [cited 2017 Feb 2]. https://webofknowledge.com/.
Berto A, da Silva AF, Visentainer JV, Matsushita M, de Souza NE. Proximate compositions, mineral contents and fatty acid compositions of native Amazonian fruits. Food Res Int. 2015;77:441–9. doi:10.1016/j.foodres.2015.08.018.
Naozuka J, Carvalho Vieira E, Nascimento AN, Oliveira PV. Elemental analysis of nuts and seeds by axially viewed ICP OES. Food Chem. 2011;124:1667–72. doi:10.1016/j.foodchem.2010.07.051.
Oliveira VB, Yamada LT, Fagg CW, Brandão MGL. Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res Int. 2012;48:170–9. doi:10.1016/j.foodres.2012.03.011.
Costa T, Jorge N. Characterization and fatty acids profile of the oils from Amazon nuts and walnuts: characterization and fatty acids profile of the oilseeds. Nutr Food Sci. 2012;42:279–87. doi:10.1108/00346651211248647.
Queiroga Neto V, Bora PS, Diniz ZN, Cavalheiro JMO, Queiroga KF. Dipteryx lacunifera seed oil: characterization and thermal stability. Ciênc Agrotecnol. 2009;33:1601–7. doi:10.1590/S1413-70542009000600020.
da Costa PA, Ballus CA, Teixeira-Filho J, Godoy HT. Phytosterols and tocopherols content of pulps and nuts of Brazilian fruits. Food Res Int. 2010;43:1603–6. doi:10.1016/j.foodres.2010.04.025.
Wickens GE. Edible nuts. Non-wood For. Prod. Rome: Food and Agriculture Organization of the United Nations; 1995. p. 198. http://www.fao.org/docrep/018/v8929e/v8929e.pdf.
Rodrigues AB, Florence CT, Mariano-Neto E, Gaiotto FA. First microsatellite markers for Lecythis pisonis (Lecythidaceae), an important resource for Brazilian fauna. Conserv Genet Resour. 2015;7:437–9. doi:10.1007/s12686-014-0390-6.
Vallilo MI, Tavares M, Aued-Pimentel S, Campos NC. Neto JMM. Lecythis pisonis Camb. nuts: oil characterization, fatty acids and minerals. Food Chem. 1999;66:197–200. doi:10.1016/S0308-8146(99)00040-0.
Carvalho MG, Costa JMC, Souza VAB, Maia GA. Avaliação dos parâmetros físicos e nutricionais de amêndoas de sapucaia and castanha-do-gurguéia. Rev Ciênc Agron. 2008;39:517–23.
de Carvalho IMM, Queirós LD, Brito LF, Santos FA, Bandeira AVM, de Souza AL, et al. Caracterização química da castanha de sapucaia (Lecythis pisonis Cambess.) da região da zona da mata Mineira. Biosci J. 2012;28:971–7.
Denadai SMS, Hiane PA, Marangoni S, Baldasso PA, Miguel AMRDO, Macedo MLR. In vitro digestibility of globulins from sapucaia (Lecythis pisonis Camb.) nuts by mammalian digestive proteinases. Ciênc Tecnol Aliment. 2007;27:535–43.
Brandão MS, Pereira SS, Lima DF, Oliveira JPC, Ferreira ELF, Chaves MH, et al. Antinociceptive effect of Lecythis pisonis Camb. (Lecythidaceae) in models of acute pain in mice. J Ethnopharmacol. 2013;146:180–6.
Silva LL, Gomes BS, Sousa-Neto BP, Oliveira JPC, Ferreira ELF, Chaves MH, et al. Effects of Lecythis pisonis Camb. (Lecythidaceae) in a mouse model of pruritus. J Ethnopharmacol. 2012;139:90–7. doi:10.1016/j.jep.2011.10.023.
Ferreira ÉLDF, Mascarenhas TS, Oliveira JPDC, Chaves MH, Araújo BQ, Cavalheiro AJ. Phytochemical investigation and antioxidant activity of extracts of Lecythis pisonis. Camb J Med Plants Res. 2014;8:353–6016. doi:10.5897/JMPR2013.5153.
Vieira MEB, Vasconcelos IM, Machado OLT, Gomes VM, Carvalho ADO. Isolation, characterization and mechanism of action of an antimicrobial peptide from Lecythis pisonis seeds with inhibitory activity against Candida albicans. Acta Biochim Biophys Sin. 2015;47:716–29. doi:10.1093/abbs/gmv071.
Samyn P, Schoukens G, Vonck L, Stanssens D, Van Den Abbeele H. Quality of Brazilian vegetable oils evaluated by (modulated) differential scanning calorimetry. J Therm Anal Calorim. 2012;110:1353–65. doi:10.1007/s10973-011-2132-2.
Teixeira GL, Züge LCB, Silveira JLM, Scheer ADP, Ribani RH. The impact of polyoxyethylene sorbitan surfactants in the microstructure and rheological behaviour of emulsions made with melted fat from Cupuassu (Theobroma grandiflorum). J Surfactants Deterg. 2016;19:725–38. doi:10.1007/s11743-016-1820-0.
AOCS. Official methods and recommended practices of the American OIL Chemists’ Society. 5th ed. Champaign: AOCS Press; 1997.
Mettler Toledo. Good titration practice in Karl Fischer titration. 2013. http://fr.mt.com/dam/LabDiv/Campaigns/TestingLabs2013/moisture/package/gtp-karl-fischer-EN.pdf.
Pena Muniz MA, dos Santos MNF, da Costa CEF, Morais L, Lamarão MLN, Ribeiro-Costa RM, et al. Physicochemical characterization, fatty acid composition, and thermal analysis of Bertholletia excelsa HBK oil. Pharmacogn Mag. 2015;11:147–51. doi:10.4103/0973-1296.149730.
Zhang X, Li L, Xie H, Liang Z, Su J, Liu G, et al. Comparative analysis of thermal behavior, isothermal crystallization kinetics and polymorphism of palm oil fractions. Molecules. 2013;18:1036–52. doi:10.3390/molecules18011036.
Menard KF, Sichina WJ. Prediction of solid fat index (SFI) values of food fats using DSC. Perkin Elmer Appl Note. Waltham: Perkin Elmer; 2000.
Bail S, Stuebiger G, Krist S, Unterweger H, Buchbauer G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008;108:1122–32. doi:10.1016/j.foodchem.2007.11.063.
Sánchez-Rangel JC, Benavides J, Heredia JB, Cisneros-Zevallos L, Jacobo-Velázquez DA. The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Anal Methods. 2013;5:5990–9. doi:10.1039/c3ay41125g.
Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi:10.1016/S0023-6438(95)80008-5.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi:10.1016/S0891-5849(98)00315-3.
Benzie I, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi:10.1006/abio.1996.0292.
Zielinski AAF, Haminiuk CWI, Beta T. Multi-response optimization of phenolic antioxidants from white tea (Camellia sinensis L. Kuntze) and their identification by LC-DAD-Q-TOF-MS/MS. LWT Food Sci Technol. 2016;65:897–907. doi:10.1016/j.lwt.2015.09.020.
Guedes AMM, Antoniassi R, Galdeano MC, Grimaldi R, De Carvalho MG, Wilhelm AE, et al. Length-scale specific crystalline structural changes induced by molecular randomization of pequi oil. J Oleo Sci. 2017;66:469–78. doi:10.5650/jos.ess16192.
Ghazani SM, Marangoni AG. Minor components in canola oil and effects of refining on these constituents: a review. JAOCS. J Am Oil Chem Soc. 2013;90:923–32. doi:10.1007/s11746-013-2254-8.
Van Hoed V. Phenolic compounds in seed oils. Lipid Technol. 2010;22:247–9. doi:10.1002/lite.201000063.
Miraliakbari H, Shahidi F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008;111:421–7. doi:10.1016/j.foodchem.2008.04.008.
Castelo-Branco VN, Santana I, Di-Sarli VO, Freitas SP, Torres AG. Antioxidant capacity is a surrogate measure of the quality and stability of vegetable oils. Eur J Lipid Sci Technol. 2016;118:224–35. doi:10.1002/ejlt.201400299.
Christodouleas DC, Fotakis C, Nikokavoura A, Papadopoulos K, Calokerinos AC. Modified DPPH and ABTS assays to assess the antioxidant profile of untreated oils. Food Anal Methods. 2015;8:1294–302. doi:10.1007/s12161-014-0005-6.
Blomhoff R, Carlsen MH, Andersen LF, Jacobs DR Jr. Health benefits of nuts: potential role of antioxidants. Br J Nutr. 2006;96:S52–60. doi:10.1017/BJN20061864.
Castelo-Branco VN, Torres AG. Potential application of antioxidant capacity assays to assess the quality of edible vegetable oils. Lipid Technol. 2009;21:152–5. doi:10.1002/lite.200900035.
Malacrida CR, Kimura M, Jorge N. Phytochemicals and antioxidant activity of citrus seed oils. Food Sci Technol Res. 2012;18:399–404. doi:10.3136/fstr.18.399.
Huang D, Boxin OU, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56. doi:10.1021/jf030723c.
Mišurcová L, Ambrožová J, Samek D. Seaweed lipids as nutraceuticals. Adv Food Nutr Res. 2011;64:339–55. doi:10.1016/B978-0-12-387669-0.00027-2.
Costa-Singh T, Bitencourt TB, Jorge N. Caracterização e compostos bioativos do óleo da castanha-de-cutia (Couepia edulis). Rev Inst Adolfo Lutz. 2012;71:61–8.
Orsavova J, Misurcova L, Ambrozova J, Vicha R, Mlcek J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci. 2015;16:12871–90. doi:10.3390/ijms160612871.
Siger A, Nogala-Kalucka M, Lampart-Szczapa E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J Food Lipids. 2008;15:137–49. doi:10.1111/j.1745-4522.2007.00107.x.
Queiroga Neto V, Bakke OA, Ramos CMP, Bora PS, Letelier JC, Conceição MM. Brazil Nut (Bertholletia excelsa HBK) seed kernel oil: characterization and thermal stability. Rev Bras Biol Farm. 2009;3:33–42.
Tengku-Rozaina TM, Birch EJ. Thermal oxidative stability analysis of hoki and tuna oils by differential scanning calorimetry and thermogravimetry. Eur J Lipid Sci Technol. 2016;118:1053–61. doi:10.1002/ejlt.201500310.
Gao F, Birch J. Oxidative stability, thermal decomposition, and oxidation onset prediction of carrot, flax, hemp, and canola seed oils in relation to oil composition and positional distribution of fatty acids. Eur J Lipid Sci Technol. 2016;118:1042–52. doi:10.1002/ejlt.201500208.
Gao F, Yang S, Birch J. Physicochemical characteristics, fatty acid positional distribution and triglyceride composition in oil extracted from carrot seeds using supercritical CO2. J Food Compos Anal. 2016;45:26–33. doi:10.1016/j.jfca.2015.09.004.
Li R, Huang J, Huang L, Teng J, Xia N, Wei B, et al. Comparison of GC and DSC monitoring the adulteration of camellia oil with selected vegetable oils. J Therm Anal Calorim Springer Neth. 2016;126:1735–46.
Tan CP, Che Man YB. Differential scanning calorimetric analysis of edible oils: comparison of thermal properties and chemical composition. J Am Oil Chem Soc. 2000;77:143–55. doi:10.1007/s11746-000-0024-6.
Del Río JC, Evaristo AB, Marques G, Martín-Ramos P, Martín-Gil J, Gutiérrez A. Chemical composition and thermal behavior of the pulp and kernel oils from macauba palm (Acrocomia aculeata) fruit. Ind Crops Prod. 2016;84:294–304. doi:10.1016/j.indcrop.2016.02.018.
Augusto PED, Soares BMC, Chiu MC, Gonçalves LAG. Modelling the effect of temperature on the lipid solid fat content (SFC). Food Res Int. 2012;45:132–5. doi:10.1016/j.foodres.2011.10.026.
Gila A, Jiménez A, Beltrán G, Romero A. Correlation of fatty acid composition of virgin olive oil with thermal and physical properties. Eur J Lipid Sci Technol. 2015;117:366–76. doi:10.1002/ejlt.201400078.
Wan Nik WB, Ani FN, Masjuki HH, Eng Giap SG. Rheology of bio-edible oils according to several rheological models and its potential as hydraulic fluid. Ind Crops Prod. 2005;22:249–55. doi:10.1016/j.indcrop.2005.01.005.
Sánchez-Gutiérrez CA, Casas M, Lucero MJ, Ruiz-Méndez MV. Physico-chemical and rheological characterization of olive-pomace oils. Eur J Lipid Sci Technol. 2015;117:87–91. doi:10.1002/ejlt.201300490.
Giap SGE. The hidden property of arrhenius-type relationship: viscosity as a function of temperature. J Phys Sci. 2010;21:29–39.
Xu Y, Wei C, Zhao X, Lu C, Dong C. A comparative study on microstructure, texture, rheology, and crystallization kinetics of palm-based diacylglycerol oils and corresponding palm-based oils. Eur J Lipid Sci Technol. 2016;118:1179–92. doi:10.1002/ejlt.201500369.
Rohman A, Che Man YB. Simultaneous quantitative analysis of two functional food oils, extra virgin olive oil and virgin coconut oil using FTIR spectroscopy and multivariate calibration. Int Food Res J. 2011;18:1231–5.
Guillén MD, Cabo N. Characterization of edible oils and lard by fourier transform infrared spectroscopy. Relationships between composition and frequency of concrete bands in the fingerprint region. J Am Oil Chem Soc. 1997;74:1281–6. doi:10.1002/(SICI)1097-0010(199709)75:1<1:AID-JSFA842>3.0.CO;2-R.
Zhang H, Ma J, Miao Y, Tuchiya T, Chen JY. Analysis of carbonyl value of frying oil by fourier transform infrared spectroscopy. J Oleo Sci. 2015;64:375–80. doi:10.5650/jos.ess14201.
Acknowledgements
The authors acknowledge the scholarship provided by the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil), Grant No. 1291783 (CAPES-DS), and the Graduate Program in Food Engineering (Federal University of Paraná, Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Teixeira, G.L., Ávila, S., Silveira, J.L.M. et al. Chemical, thermal and rheological properties and stability of sapucaia (Lecythis pisonis) nut oils. J Therm Anal Calorim 131, 2105–2121 (2018). https://doi.org/10.1007/s10973-017-6742-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6742-1