Abstract
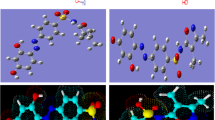
New azodye ligand (H2L) and its relative Cr(III)-, Mn(II)-, Fe(III)-, Co(II)-, Ni(II)-, Cu(II)-, Zn(II)- and Cd(II)-nanosized complexes were prepared. A new synthesized compounds were characterized using spectral (mass, IR, UV–Vis, XRD, and ESR) and analytical (elemental, molar conductance, thermal and magnetic moment measurements) tools. Infrared spectra showed that the ligand behaves as a monobasic bidentate, coordinating with central atoms through carbonyl oxygen and α-hydroxyl group. The geometrical structures of Cr(III) and Fe(III) complexes were found to be in octahedral configuration, whereas Mn(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) complexes have tetrahedral forms. XRD patterns reflect an amorphous appearance of all investigated complexes. TEM images showed nanosized particles and identical distribution over the complex surface. Molecular modeling for the drug ligand and its metal ion complexes were performed using Gaussian09 program to assert on their structural formulae. Some essential parameters were extracted using HOMO and LUMO energies. AutoDock tools 4.2 was used to simulate the interaction process with infected cell proteins to expect the experimental pathway. The inhibition activity of drug ligand and its metal ion complexes was evaluated towards different types of bacteria and fungi through in vitro antimicrobial activities. The antitumor activities of all compounds are straightened towards human liver carcinoma (HEPG2) cell lines. Fe(III) and Co(II) complexes exhibited IC50 of 2.90 and 4.23 µg mL−1, respectively, which means they are more potent anticancer drug than the standard (doxorubicin, IC50 = 4.73 µg mL−1). Therefore, the two complexes may consider promising anticancer drugs.










Similar content being viewed by others
References
Blass B. Sulfonamide derivatives and pharmaceutical applications thereof. Med Chem Lett. 2016;7:12–4.
Bhatt A, Kant R, Singh RK. Synthesis of some bioactive sulfonamide and amide derivatives of piperazine incorporating imidazo[1,2-B]pyridazine moiety. Med Chem. 2016;6:257–63.
Shah SSA, Rivera G, Ashfaq M. Recent advances in medicinal chemistry of sulfonamides. Rational design as anti-tumoral, anti-bacterial and anti-inflammatory agents. Mini Rev Med Chem. 2016;13:70–86.
Li M, Takada K, Goldsmith JI, Bernhard S. Iridium(III) bis-pyridine-2-sulfonamide complexes as efficient and durable catalysts for homogeneous water oxidation. Inorg Chem. 2016;55:518–26.
Hangan AC, Turza A, Stan RL, Sevastre B, Páll E, Cetean S, Oprean LS. Synthesis, crystal structure and characterization of new biologically active Cu(II) complexes with ligand derived from N-substituted sulfonamide. J Chem Sci. 2016;128:815–24.
Sayed AR, Youssef MM, Al-Faiyz YS. Synthesis, characterization and biological evaluation of novel thiadiazoline sulfonamides and metal complexes. J Appl Sci. 2015;15:884–93.
Sahai HK, Sekhon BS, Randhawa HS. Chemical and spectroscopic studies on the molecular complexes of antibacterial and antiamoebic drugs with picric acid and silver picrate. J Chem. 1999;52:20–3.
El-Ghamry H, Sakai K, Masaoka S, El-Baradie K, Issa R. Synthesis and characterization of the self-assembled coordination polymers of N-diaminomethylene-4-(3-formyl- 4-hydroxy-phenylazo)-benzenesulfonamide ligand. J Coord Chem. 2012;65:780–94.
Gaffer HE, Fouda MMG, Khalifa ME. Synthesis of some novel 2-amino-5-arylazothiazole disperse dyes for dyeing polyester fabrics and their antimicrobial activity. Molecules. 2016;21(122):1–10.
Khedr AM. Investigation of 3-amino-1,2,4-triazole azodye derivatives as reagents for determination of mercury(II). Chem Pap. 2008;62:541–6.
Santra BK, Lahiri GK. Cobalt-mediated direct and selective aromatic thiolation in the complex [CoIII(o-SC6H4N=NC5H4N)2]ClO4. Synthesis, spectroscopic, characterization and electron-transfer properties. J Chem Soc Dalton Trans. 1997;11:1883–8.
Misra TK, Das D, Sinha C, Ghosh P, Pal CK. Chemistry of azoimidazoles: synthesis, spectral characterization, electrochemical studies, and x-ray crystal structures of isomeric dichloro bis[1-alkyl-2-(arylazo)imidazole] complexes of ruthenium(II). Inorg Chem. 1998;37:1672–8.
Idemudia OG, Sadimenko AP, Afolayan AJ, Hosten EC. Synthesis and characterization of bioactive acylpyrazolone sulfanilamides and their transition metal complexes: single crystal structure of 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one sulfanilamide. Bioinorg Chem Appl. 2015;2015:1–14.
Saad FA, Khedr AM. Greener solid state synthesis of nano-sized mono and homo bi-nuclear Ni(II), Co(II), Mn(II), Hg(II), Cd(II) and Zn(II) complexes with new sulfa ligand as a potential antitumour and antimicrobial agents. J Mol Liq. 2017;231:572–9.
Abbas AK, Kadhim RS. Metal complexes of proline-azodyes, synthesis, characterization, dying performance and antibacterial activity studies. Orient J Chem. 2017;33:402–17.
El-Ansary AL, Abd El-Fattah HM, Abdel-Kader NS, Farghaly AM. Synthesis and characterization of some sulfadrugs azodyes, potentiometric studies of the synthesized dyes and their Fe(III) complexes. Commun Inorg Synth. 2013;1:16–8.
El-Mossalamy EH. Potentiometric and spectroscopic studies of sulfonamide azo-dye complexes with some transition metal ions and uranium. Port Electrochim Acta. 2009;27:143–52.
El-Sonbati AZ, Diab MA, El-Halawany MM, Salam NE. Polymer complexes: XLXII-interplay of coordination π–π stacking and hydrogen bonding in supramolecular assembly of sulpha drug derivatives-N, S: N, O complexes. Spectrochim Acta A. 2010;77:755–66.
Modhavadiya VA. Synthesis, characterization, spectral studies, biocidal activities of Fe(II) and Cu(II) complexes of azodye ligand derived from sulfamethoxazole and substituted p-Cresol. Orient J Chem. 2012;28:921–5.
Roblin RO, Williams JH, Winnek PS, English JP. Chemotherapy. II. Some sulfanilamido heterocycles. J Am Chem Soc. 1940;62:2002–5.
Saad FA. Synthesis and characterization of the self-assembled coordination polymers of N-diaminomethylene-4-(3-formyl-4-hydroxy-phenylazo)-benzenesulfonamide ligand. Spectrochim Acta A. 2014;128:386–92.
Al-Ashqer S, Abou-Melha KS, Al-Hazmi GAA, Saad FA, El-Metwaly NM. Spectral studies on a series of metal ion complexes derived from pyrimidine nucleus, TEM, biological and γ-irradiation effect. Spectrochim Acta A. 2014;132:751–61.
Saad FA. Co-ordination chemistry of some first row transition metal complexes with multi-dentate ligand (1- benzoyl-3-(4-methylpyridin-2-yl) thiourea), spectral, electrochemical and X-ray single crystal studies. Int J Electrochem Sci. 2014;9:4761–75.
Saad FA, Khedr AM. Azo-azomethine ligands with N2O2 donor atom sets and their binuclear UO2(II) complexes: synthesis, characterization and biological activity. Bulg Chem Commun. 2015;47:654–63.
Shah RK, Abou-Melha KS, Saad FA, Yousef T, Al- Hazmi GAA, Elghalban MG, Khedr AM, El-Metwaly N. Elaborated studies on nano-sized homo-binuclear Mn(II), Fe(III), Co(II), Ni(II), and Cu(II) complexes derived from N2O2 Schiff base, thermal, molecular modeling, drug-likeness, and spectral. J Therm Anal Calorim. 2016;131:731–43.
Khedr AM, Saad FA. Synthesis, structural characterization, and antimicrobial efficiency of sulfadiazine azo-azomethine dyes and their bi-homonuclear uranyl complexes for chemotherapeutic use. Turk J Chem. 2015;39:267–80.
Frisch MJ, et al. Gaussian 09, Revision D. Wallingford: Gaussian, Inc; 2010.
Dennington R, Keith T, Millam J. GaussView, Version 4.1.2, Semichem Inc, Shawnee Mission, KS; 2007.
Halgren TA. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem. 1996;17:490–519.
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem. 1998;19:1639–62.
Solis FJ, Wets RJB. Minimization by random search techniques. Math Oper Res. 1981;6:19–30.
Scott AC. Laboratory control of antimicrobial therapy. In: Ridgway JG, et al., editors. Practical medical microbiology. 13th ed. Churchill Livingestone: Edinburgh; 1981. p. 161–81.
Wilson AP. Cytotoxicity and viability assays in animal cell culture: a practical approach. In: Masters JRW, editor. vol. 1, 3rd ed. Oxford: Oxford university press; 2000.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Onwudiwe DC, Ekennia AC, Hosten E. Syntheses, characterization, and antimicrobial properties of nickel(II) dithiocarbamate complexes containing NiS4 and NiS2PN moieties. J Coord Chem. 2016;69:2454–68.
Singh BK, Mishra P, Garg BS. Nickel(II) complexes of new polydentate carboxyamide: spectroscopic, kinetics of thermal decomposition and X-ray powder diffraction studies. Transit Met Chem. 2007;32:603–14.
Khedr AM, Jadon S, Kumar V. Synthesis, spectral analysis, and molecular modeling of bioactive Sn(II)-complexes with oxadiazole Schiff bases. J Coord Chem. 2011;64:1351–9.
Issa RM, El-baradie KY, El-Wakiel NA. UV/Vis, IR and 1H NMR spectrophotometric studies of some bisazo-dianil compounds based on bis-(hydroxy, formyl) phenylazo phenylene and anthranilic acid. Spectrochim Acta A. 2004;60:2883–9.
Nakamoto K. Infrared spectra of inorganic and coordination compounds. New York: Wiley; 1986.
Mohamed GG, Gad-Elkareem MAM. Synthesis, characterization and thermal studies on metal complexes of new azo compounds derived from sulfa drugs. Spectrochim Acta A. 2007;68:1382–7.
Issa RM, Azim SA, Khedr AM, Draz DF. Synthesis, characterization, thermal, and antimicrobial studies of binuclear metal complexes of sulfa-guanidine Schiff bases. J Coord Chem. 2009;62:1859–70.
El-Wakiel N, Khedr AM, Mansour R. Synthesis, spectral and thermal analyses of novel bi- and polyhomonuclear complexes of pyrimidine bisazo-dianil derivatives. Chin J Chem. 2010;28:463–70.
Cotton FA, Wilkinson G, Murillo CA, Bochmann M. Advanced inorganic chemistry. 6th ed. New York: Wiley; 1999.
Alaghaz AMA, Bayoumi HA, Ammar YA, Aldhlmani SA. Synthesis, characterization, and antipathogenic studies of some transition metal complexes with N, O-chelating Schiff’s base ligand incorporating azo and sulfonamide Moieties. J Mol Struct. 2013;1035:383–99.
Price ER, Wasson JR. Complexes with sulfur and selenium donors-X chromium(III) piperidyldithiocarbamates. J Inorg Nucl Chem. 1974;36:67–71.
El-Asmy AA, Rakha TH, Abdel-Rhman MH, Hassanien MM, Al-Mola AS. Synthesis, spectral, thermal and biological studies on N_(2,4-dinitrophenyl)-2-mercaptoacetohydrazide and its metal complexes. Spectrochim Acta A. 2015;136:1718–27.
El-Megharbel S, El-Metwaly N, Refat M. Synthesis of uranyl(II), vanadyl(II) and zirconylurate complexes, spectral, thermal and biological studies. Spectrochim Acta A. 2015;149:263–70.
Gaber M, El-Wakiel NA, El-Ghamry H, Fathalla SK. Synthesis, spectroscopic characterization, DNA interaction and biological activities of Mn(II), Co (II), Ni (II) and Cu (II) complexes with [(1H-1, 2, 4-triazole-3-ylimino) methyl] naphthalene-2-ol. J Mol Struct. 2014;1076:251–61.
El-wakiel N, El-Keiy M, Gaber M. Synthesis, spectral, antitumor, antioxidant and antimicrobial studies on Cu(II), Ni(II) and Co(II) complexes of 4-[(1H-Benzoimidazol-2-ylimino)-methyl]-benzene-1,3-diol. Spectrochim Acta A. 2015;147:117–23.
Al-Neaimi M, Al-Khuder MM. Synthesis, characterization and extraction studies of some metal (II) complexes containing (hydrazoneoxime and bis-acylhydrazone) moieties. Spectrochim Acta A. 2013;105:365–73.
Al-Dawood AY, El-Metwaly NM, El-Ghamry HA. Molecular docking and DFT studies on some nano-meter binuclear complexes derived from hydrazine-carbothioamide ligand, synthesis, thermal, kinetic and spectral characterization. J Mol Liq. 2016;220:311–23.
Alaghaz AMA, Ammar RA. New dimeric cyclodiphosph(V)azane complexes of Cr(III), Co(II), Ni(II), Cu(II), and Zn(II): preparation, characterization and biological activity studies. Eur J Med Chem. 2010;45:1314–22.
Kumar DN, Garg BS. Synthesis and spectroscopic studies of complexes of zinc(II) with N2O2 donor groups Spectrochim. Acta A. 2006;64:141–7.
Park HI, Ming LJ. The mechanistic role of the coordinated tyrosine in astacin. J Inorg Biochem. 1998;78:57–62.
Hathaway BJ, Billing DE. Electronic properties and stereochemistry of mononuclear complexes of the copper(II) ion. Coord Chem Rev. 1970;5:143–207.
Kivelson D, Neiman R. ESR studies on the bonding in copper complexes. J Chem Phys. 1961;35:149–55.
Maurya RC, Rajput S. Oxovanadium (IV) complexes of bioinorganic and medicinal relevance: synthesis, characterization, and 3D molecular modeling and analysis of some oxovanadium (IV) complexes involving O, O-donor environment. J Mol Struct. 2004;687:35–44.
Wellman JA, Hulsbergen FB, Verbiest J, Reedijk J. Influence of alkyl chain length in N-alkyl imidazoles upon the complex formation with transition-metal salts. J Inorg Nucl Chem. 1978;40:143–7.
Yokoi H, Addison AW. Spectroscopic and redox properties of pseudotetrahedralcopper(II) complexes their relation to copper proteins. Inorg Chem. 1977;16:1341–9.
Fahem AA. Comparative studies of mononuclear Ni(II) and UO2(II) complexes having bifunctional coordinated groups: synthesis, thermal analysis, X-ray diffraction, surface morphology studies and biological evaluation. Spectrochim Acta A. 2012;88:10–22.
Saad FA, Elghalban MG, El-Metwaly N, El-Ghamry H, Khedr AM. Density functional theory/B3LYP study of nanometric 4-(2,4-dihydroxy-5-formylphen-1-ylazo)-N-(4-methylpyrimidin-2-yl)benzenesulfonamide complexes: quantitative structure–activity relationship, docking, spectral and biological investigations. Appl Organomet Chem. 2017;. doi:10.1002/aoc.3721.
Hui JK, MacLachlan MJ. Metal-containing nanofibers via coordination chemistry. Coord Chem Rev. 2010;254:2363–90.
Salimi A, Hallaj R, Soltanian S. Immobilization of hemoglobin on electrodeposited cobalt-oxide nanoparticles: direct voltammetry and electrocatalytic activity. Biophys Chem. 2007;130:122–31.
Badea M, Emandi A, Marinescu D, Cristurean E, Olar R, Braileanu A, Budrugeac P, Segal E. Thermal stability of some azo-derivatives and their complexes. J Therm Anal Calorim. 2003;72:525–31.
Ray RK, Kauffman GR. EPR spectra and covalency of bis(amidinourea/o-alkyl-1- amidinourea)copper(II) complexes, Part II. Properties of the [CuN4] 2-chromophore. Inorg Chem Acta. 1990;173:207–2014.
Fleming I. Frontier orbitals and organic chemical reactions. London: Wiley; 1976.
Chikate RC, Padhye SB. Transition metal quinone–thiosemicarbazone complexes 2: magnetism, ESR and redox behavior of iron(II), iron(III), cobalt(II) and copper(II) complexes of 2-thiosemicarbazido-1, 4-naphthoquinone. Polyhedron. 2005;24:1689–700.
Sagdinc S, Köksoy B, Kandemirli F. BayariSH. Theoretical and spectroscopic studies of 5-fluoro-isatin-3-(N-benzylthiosemicarbazone) and its zinc(II) complex. J Mol Struct. 2009;917:63–70.
George RF. Stereoselective synthesis and QSAR study of cytotoxic 2-(4-oxo-thiazolidin-2-ylidene)-2-cyano-N-arylacetamides. Eur J Med Chem. 2012;47:377–86.
Tripathi K, Muttineni R, Singh SK. Extra precision docking, free energy calculation and molecular dynamics simulation studies of CDK2 inhibitors. J Theo Bio. 2013;334:87–100.
Amer S, El-Wakiel N, El-Ghamry H. Synthesis, spectral, antitumor and antimicrobial studies on Cu (II) complexes of purine and triazole Schiff base derivatives. J Mol Struct. 2013;1049:326–35.
Phaniband MA, Dhumwad SD. Synthesis, characterization and biological studies of CoII, NiII, CuII and ZnII complexes of Schiff bases derived from 4-substituted carbostyrils[quinolin2(1H)-ones]. Transit Met Chem. 2007;32:1117–25.
Yadav LDS, Singh S. Synthesis and antiviral activity of acrylic C-nucleoside incorporating thiazolo-1,3,4-thiadiazole. Ind J Chem B. 2001;40:440–2.
Singh BK, Jetley UK, Sharma RK, Garg BS. Synthesis, characterization and biological activity of complexes of 2-hydroxy-3,5-dimethylacetophenoneoxime (HDMAOX) with copper(II), cobalt(II), nickel(II) and palladium(II). Spectrochim Acta A. 2007;68:63–73.
Sultan AS, Brim H, Sherif ZA. Co-overexpression of Janus kinase 2 and signal transducer and activator of transcription 5a promotes differentiation of mammary cancer cells through reversal of epithelial-mesenchymal transition. Cancer Sci. 2008;2:272–9.
Etaiw SH, Amer SA, El-Bendary MMA. Mixed valence copper cyanide 3D-supramolecular coordination polymer containing 1,10-phenathorline ligand as a potential antitumor agent, effective catalyst and luminescent material. J Inorg Organomet Polym. 2011;21:662–9.
Riera X, Moreno V, Ciudad CJ, Noe V, Font-Bardía M, Solans X. Bioinorg complexes of Pd(II) and Pt(II) with 9-aminoacridine: Reactions with DNA and study of their antiproliferative activity. Chem Appl. 2007;2007:98732–47.
Shier WT. Mammalian cell culture on $5 a day: a lab manual of low cost methods. Los Banos: University of the Philippines; 1991.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saad, F.A., Al-Fahemi, J.H., El-Ghamry, H. et al. Elaborated spectral, modeling, QSAR, docking, thermal, antimicrobial and anticancer activity studies for new nanosized metal ion complexes derived from sulfamerazine azodye. J Therm Anal Calorim 131, 1249–1267 (2018). https://doi.org/10.1007/s10973-017-6598-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6598-4