Abstract
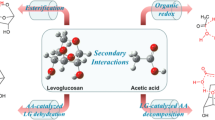
During biomass fast pyrolysis process, the interactions among biomass components will affect the pyrolytic products distribution. In this study, d-glucose and a β-O-4 type lignin model dimer (LMD, 1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)propane-1,3-diol) were selected as the model compounds of cellulose and lignin. The interaction characteristics and mechanism during their fast co-pyrolysis process were investigated by combined pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) experiments and density functional theory (DFT) calculations. The Py–GC/MS results indicated that during fast co-pyrolysis process, the presence of LMD significantly decreased the formation of levoglucosan (LG) from d-glucose, while promoted the formation of linear carbonyls and furans. Meanwhile, the presence of d-glucose enhanced the decomposition of LMD to generate phenolic compounds. The DFT calculations revealed that d-glucose would interact with a homolysis radical of LMD to form a ten-membered ring transition state. The formed complex transition state changed the energy barriers of certain pyrolytic reactions of d-glucose and LMD, thus affecting the pyrolytic products distribution.






Similar content being viewed by others
References
Bridgwater AV. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy. 2012;38:68–94.
Shen DK, Jin W, Hu J, Xiao R, Luo KH. An overview on fast pyrolysis of the main constituents in lignocellulosic biomass to valued-added chemicals: structures, pathways and interactions. Renew Sustain Energy Rev. 2015;51:761–74.
Huang L, Ding T, Liu R, Cai J. Prediction of concentration profiles and theoretical yields in lignocellulosic biomass pyrolysis. J Therm Anal Calorim. 2015;120:1473–82.
Tereas SP, Salvador N, Jorge LB, Javier TS, Ramon A. Thermogravimetric analysis of wood, holocellulose, and lignin from five wood species. J Therm Anal Calorim. 2012;109:1163–7.
Pasangulapati V, Ramachandriya KD, Kumar A, Wilkins MR, Jones CL, Huhnke RL. Effects of cellulose, hemicellulose and lignin on thermochemical conversion characteristics of the selected biomass. Bioresour Technol. 2012;114:663–9.
Yang HP, Yan RY, Chen HP, Lee DH, Zheng CG. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–8.
Lu Q, Yang XC, Dong CQ, Zhang ZF, Zhang XM, Zhu XF. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: analytical Py–GC/MS study. J Anal Appl Pyrolysis. 2011;92:430–8.
Shen DK, Gu S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour Technol. 2009;100:6496–504.
Huang XY, Cheng DG, Chen FQ, Zhan XL. Reaction pathways of hemicellulose and mechanism of biomass pyrolysis in hydrogen plasma: a density functional theory study. Renew Energy. 2016;96:490–7.
Cao XF, Zhong LX, Peng XW, Sun SN, Li SM, Liu SJ, Sun RC. Comparative study of the pyrolysis of lignocellulose and its major components: characterization and overall distribution of their biochars and volatiles. Bioresour Technol. 2014;155:21–7.
Wang SR, Ru B, Dai GX, Sun WX, Qiu KZ, Zhou JS. Pyrolysis mechanism study of minimally damaged hemicellulose polymers isolated from agricultural waste straw samples. Bioresour Technol. 2015;190:211–8.
Zhang JJ, Jiang XY, Ye XN, Chen L, Lu Q, Wang XH, Dong CQ. Pyrolysis mechanism of a β-O-4 type lignin dimer model compound: a joint theoretical and experimental study. J Therm Anal Calorim. 2016;123:501–10.
Bartkowiak M, Zakrzewski R. Thermal degradation of lignins isolated from wood. J Therm Anal Calorim. 2004;77:295–304.
Beste A, Buchanan AC. Kinetic analysis of the phenyl-shift reaction in β-O-4 lignin model compounds: a computational study. J Org Chem. 2011;76:2195–203.
Kim YM, Jae JH, Myung SY, Sung BH, Dong JI, Park YK. Investigation into the lignin decomposition mechanism by analysis of the pyrolysis product of Pinus radiate. Bioresour Technol. 2016;219:371–7.
Wang SR, Ru B, Lin HZ, Sun WX, Luo ZY. Pyrolysis behaviors of four lignin polymers isolated from the same pine wood. Bioresour Technol. 2015;182:120–7.
Liu Q, Zhong Z, Wang SR, Luo ZY. Interactions of biomass components during pyrolysis: a TG-FTIR study. J Anal Appl Pyrolysis. 2011;90:213–8.
Yang HP, Yan RY, Chen HP, Zheng CG, Lee DH. In-depth investigation of biomass pyrolysis based on three major components: hemicellulose, cellulose and lignin. Energy Fuels. 2006;20:388–93.
Lopez R, Fernandez C, Gomez X, Martinez O, Sanchez ME. Thermogravimetric analysis of lignocellulosic and microalgae biomasses and their blends during combustion. J Therm Anal Calorim. 2013;114:295–305.
Long YQ, Zhou H, Meng AH, Li QH, Zhang YG. Interactions among biomass components during co-pyrolysis in (macro) thermogravimetric analyzers. Korean J Chem Eng. 2016;33:2638–43.
Stefanidis DS, Kalogiannis GK, Iliopoulou FE, Michailofa MC, Pilavachi AP, Lappas AA. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J Anal Appl Pyrolysis. 2014;105:143–50.
Hilbers TJ, Wang ZH, Pecha B, Westerhof RJM, Kersten SRA, Pelaez-Samaniego MR, Garcia-Perez M. Cellulose-lignin interactions during slow and fast pyrolysis. J Anal Appl Pyrolysis. 2015;114:197–207.
Hosoya T, Kawamoto H, Saka S. Cellulose-hemicellulose and cellulose-lignin interactions in wood pyrolysis at gasification temperature. J Anal Appl Pyrolysis. 2007;80:118–25.
Hosoya T, Kawamoto H, Saka S. Solid/liquid- and vapor-phase interactions between cellulose- and lignin-derived pyrolysis products. J Anal Appl Pyrolysis. 2009;85:237–46.
Wu S, Shen DK, Hu J, Zhang HY, Xiao R. Cellulose–lignin interactions during fast pyrolysis with different temperatures and mixing methods. Biomass Bioenergy. 2016;90:209–17.
Wang SR, Guo XJ, Wang KG, Luo ZY. Influence of the interaction of components on the pyrolysis behavior of biomass. J Anal Appl Pyrolysis. 2011;91:183–9.
Zhang J, Choi YS, Yoo CG, Kim TH, Brown RC, Shanks BH. Cellulose–hemicellulose and cellulose–lignin interactions during fast pyrolysis. ACS Sustain Chem Eng. 2015;3:293–301.
Ye XN, Lu Q, Li WT, Gao P, Hu B, Zhang ZB, Dong CQ. Selective production of nicotyrine from catalytic fast pyrolysis of tobacco biomass with Pd/C catalyst. J Anal Appl Pyrolysis. 2016;117:88–93.
Frisch MJ, Trucks GW, Schlegel HB. Gaussian 09, Revision D.01. Pittsburgh: Gaussian, Inc.; 2003.
Gonzalez C, Schlegel HB. An improved algorithm for reaction path following. J Chem Phys. 1989;90:2154–61.
Paine JB, Pithawalla YB, Naworal JD. Carbohydrate pyrolysis mechanisms from isotopic labeling. Part 2. The pyrolysis of d-glucose: general disconnective analysis and the formation of C-1 and C-2 carbonyl compounds by electrocyclic fragmentation mechanisms. J Anal Appl Pyrolysis. 2008;82:10–41.
Paine JB, Pithawalla YB, Naworal JD. Carbohydrate pyrolysis mechanisms from isotopic labeling. Part 3. The pyrolysis of d-glucose: formation of C-3 and C-4 carbonyl compounds and a cyclopentenedione isomer by electrocyclic fragmentation mechanisms. J Anal Appl Pyrolysis. 2008;82:42–69.
Paine JB, Pithawalla YB, Naworal JD. Carbohydrate pyrolysis mechanisms from isotopic labeling Part 4. The pyrolysis of d-glucose: the formation of furans. J Anal Appl Pyrolysis. 2008;83:37–63.
Carlson TR, Jae J, Lin YC, Tompsett GA, Huber GW. Catalytic fast pyrolysis of glucose with HZSM-5: the combined homogeneous and heterogeneous reactions. J Catal. 2010;270:110–24.
Lu Q, Tian HY, Hu B, Jiang XY, Dong CQ, Yang YP. The pyrolysis mechanism of holocellulose-based monosaccharides: the formation of the hydroxyacetaldehyde. J Anal Appl Pyrolysis. 2016;120:15–26.
Zhang YY, Liu C, Xie H. Mechanism studies on β-d-glucopyranose pyrolysis by density functional theory methods. J Anal Appl Pyrolysis. 2014;105:23–34.
Britt PF, Buchanan AC, Cooney MJ, Martineau DR. Flash vacuum pyrolysis of methoxy-substituted lignin model compounds. J Org Chem. 2000;65:1376–89.
Assary RS, Curtiss LA. Thermochemistry and reaction barriers for the formation of levoglucosenone from cellobiose. ChemCatChem. 2012;4:200–5.
Hosoya T, Nakao Y, Sato H, Kawamoto H, Sakaki S. Thermal degradation of methyl β-d-glucoside. A theoretical study of plausible reaction mechanisms. J Org Chem. 2009;74:6891–4.
Zhang XL, Yang WC, Dong CQ. Levoglucosan formation mechanisms during cellulose pyrolysis. J Anal Appl Pyrolysis. 2013;104:19–27.
Beste A, Buchanan AC. Substituent effects on the reaction rates of hydrogen abstraction in the pyrolysis of phenethyl phenyl ethers. Energy Fuels. 2010;24:2857–67.
Kawamoto H, Watanabe T, Saka S. Strong interactions during lignin pyrolysis in wood—a study by in situ probing of the radical chain reactions using model dimers. J Anal Appl Pyrolysis. 2015;113:630–7.
Acknowledgements
The authors thank the National Natural Science Foundation of China (51576064, 51676193), Beijing Nova Program (Z171100001117064), Beijing Natural Science Foundation (3172030), Foundation of State Key Laboratory of Coal Combustion (FSKLCCA1706) and Fundamental Research Funds for the Central Universities (2016YQ05, 2015ZZD02) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ye, Xn., Lu, Q., Jiang, Xy. et al. Interaction characteristics and mechanism in the fast co-pyrolysis of cellulose and lignin model compounds. J Therm Anal Calorim 130, 975–984 (2017). https://doi.org/10.1007/s10973-017-6465-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6465-3