Abstract
The paper presents the analysis of thermal stability and decomposition of Al2O3 and (Ba,Sr)TiO3 green bodies as well as commercially available organic additives which are used in shaping of ceramics by colloidal processing. The first analyzed ceramic material was Al2O3 green body obtained by gelcasting. The second examined material was elastic ceramic–polymer composite based on ferroelectric powder Ba0.8Sr0.2TiO3 with NiO obtained by tape casting method. This flexible and durable material is characterized by high value of dielectric constant and finds the application in devices operating at very high frequencies up to sub-THz. Most organic additives are indispensable just in forming step but must be then eliminated in order to obtain pure ceramic phase, additionally some of them have to be stable in a wide temperature range. The analyzed organic substances were as follows: 2-carboxyethyl acrylate, SYNTRAN 8250, DURAMAX D-3005, DURAMAX B-1000. The DTA/TG measurements coupled with mass spectrometry allowed to gain new knowledge concerning degradation of selected organic additives, thermal stability of ceramic–polymer composite and type of gases released from samples during thermal treatment.
Similar content being viewed by others
Introduction
Nowadays, the innovative ceramics represent a diverse group of materials which are increasingly used in electronics, space industry, medicine, and as protection devices [1–5]. One of the main stages in the preparation of ceramic elements is forming process including an appropriate selection of organic additives. Literature data concerning shaping methods show that ceramic materials are recently willingly fabricated by colloidal processes [6, 7]: gelcasting [8], slip casting [9], tape casting [10, 11] mechanical foaming [12], direct coagulation casting [13], dip coating [14], etc.
One of the attractive colloidal shaping methods is gelcasting which combines traditional casting from slips with polymer chemistry. Gelcasting consists in the organic monomer polymerization reaction inside ceramic slurry. In the process the macromolecular gel network, which results from the in situ polymerization of organic monomers added to the slurry, is created and holds ceramic particles in the desired shape. Gelcasting has more advantages than “dry methods.” This is due to the ability to control and influence the processes which take place in ceramic slurry, mainly the interactions between components in ceramic suspensions. In colloidal processing, it is easier to eliminate some unfavorable phenomena, like agglomeration of the particles during milling, what significantly reduces the number of structural defects and increases the homogeneity and density of samples. The main advantage of gelcasting, in comparison with other shaping methods, is high mechanical strength of green bodies. It is caused by the creation of strong polymeric network which holds ceramic particles together [15, 16].
The next and the key stage during preparation of ceramic materials is sintering process. The organic additives used during the preparation of green bodies are subsequently burned out during thermal treatment. The conditions of sintering should be selected individually for each type of the material what is possible thanks to DTA/TG measurements. The important information from thermal analysis is the temperature at which decomposition of organics ends. Additionally, the coupling of DTA apparatus with mass spectrometry allows to observe type of gases released to the atmosphere during decomposition of any substances [17–24].
The second shaping technique based on colloidal processing is tape casting which is widely used in the production of thin, flexible tapes. The homogenized slurry passes beneath the knife’s edge (commonly referred to as a doctor blade) which controls the thickness of the tapes. A combination of an elastic polymer with a ceramic powder having ferroelectric properties allows one to use this type of composite in devices operating at very high frequencies up to sub-terahertz [25]. Barium strontium titanate (BST) is a typical example of a ferroelectric material which is applied in microwave technologies [26–28]. The described in the paper ceramic–polymer composites are characterized by suitable durability, flexibility, homogeneous surface, and resistance to vibration, additionally they are environmentally friendly. For this reason, these materials are competitive in designing many different tunable devices such as antennas, phase shifters and filters which can operate at wide temperature range. Therefore, from the application point of view another important parameter is thermal stability of the composite which can be investigated by DTA/TG measurements.
The aim of the research was the preparation of ceramic–polymer composites based on BST and Al2O3 green bodies by colloidal shaping of ceramics (gelcasting and tape casting) and then to examine their behavior during thermal treatment. Additionally, the thermal characteristics of organic additives used in the shaping process was performed, that is commercially available dispersing agents SYNTRAN 8250 and DURAMAX D-3005, organic monomer 2-carboxyethyl acrylate and polymeric binder DURAMAX B-1000. The carried out research allowed to gain new knowledge about thermal stability of these materials and type of gaseous products released from the samples.
Materials and experimental procedure
The first ceramic powder used in the research was α-Al2O3 A16SG (Almatis) of the average particle size D 50 = 0.5 μm, specific surface area of 7.5 m2 g−1 and density of 3.90 g cm−3. As the organic monomer commercially available 2-carboxyethyl acrylate (CEA, Sigma-Aldrich) was used, the role of the dispersing agent was played by SYNTRAN 8250 (Interpolymer Company). According to the information given by the supplier SYNTRAN is a medium molecular weight aqueous solution based on polyacrylic acid homopolymer and was used in the form of 40% aqueous solution. Alumina green bodies have been obtained by gelcasting method, according to the procedure described elsewhere [8]. The concentration of Al2O3 powder was 50 vol%, while the concentrations of SYNTRAN 8250 and CEA equaled 1.4 and 4.0 mass%, respectively, regarding ceramic powder content.
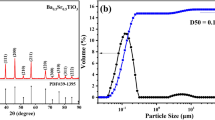
The second used ceramic powder was Ba0.8Sr0.2TiO3 doped by NiO of the average particle size from 0.5 to 0.9 µm. Barium strontium titanate was prepared using solid-state synthesis process at 1350 °C for 2 h. The materials used in the synthesis were: BaCO3, SrCO3, and TiO2. The addition of NiO was 3 mol%. In the literature, there is information about thermal characterization of BST powder during sol–gel synthesis process [27]. The results show that in case of sol–gel-derived barium titanate there exist several intermediate phases prior to the transformation of the amorphous phase into the perovskite phase. On the basis of thermal analysis, the temperature of calcination was estimated at 850 °C. Thermal analysis is therefore a useful tool in the synthesis of ceramic powders. In this work, authors have concentrated on the shaping process using BST as the main component. The elastic ceramic–polymer composite has been obtained by tape casting method in which as a dispersing agent DURAMAX D-3005 (DOW) and as a binder DURAMAX B-1000 (DOW) were used. DURAMAX D-3005 is the ammonium salt of acrylic homopolymer and was supplied as a 35% aqueous solution. DURAMAX B-1000 is an aqueous emulsion (55%) used for enhancing green strength and improving flexibility of ceramic parts. The concentration of BST powder was 50 vol%, while the concentrations of DURAMAX D-3005 and DURAMAX B-1000 equaled 1.5 and 15 mass%, respectively, regarding ceramic powder content. The thin sample was prepared using MSK-AFA-III-Automatic Thick Film Coater (MTI Corporation). The thickness of the composite tape was 180 μm. The aim of the DTA/TG measurements was to estimate thermal stability of obtained BST tape.
DTA/TG measurements were carried out by using Netzsch Jupiter STA 449C coupled with the mass spectrometer Netzsch QMS 403C Aeolos. The quantities of polymeric samples taken to measurements equaled from 0.01 to 0.15 g (depending on the sample density and molecular weight). They have been covered by calcinated (non-reactive) Al2O3 powder in the quantity of 0.3 g in order to prevent the polymer creeping from the crucible. The mass of Al2O3 sample was 0.3308 g, while the mass of BST sample was 0.1929 g. The heating rate was 5 °C min−1 and the final temperature was 1000 °C. The measurements were performed in the constant flow of two gases: argon—10 mL min−1 (protective gas) and synthetic air (75:25 N2:O2)—60 mL min−1. Mass spectrometer was set to detect m/z values in mass range 10–300.
Results and discussion
Two types of materials have been obtained. The first one was BST ceramic–polymer composite (Fig. 1a). It is a highly elastic sample due to the application of DURAMAX B-1000 as a binder. The second material was Al2O3 green body (Fig. 1b) made by gelcasting technique. Al2O3 green samples exhibit high mechanical strength (ca. 3.77 MPa) as has been wider described in previous work [8].
DTA/TG/DTG curves of thermal degradation of polymer based on 2-carboxyethyl acrylate (shown in Fig. 2a), indicate that the total mass loss was 93%, what means that almost all of organic phase has decomposed. Polymer decomposition goes in two main stages according to TG curve. It begins at ca. 173 °C and ends at ca. 585 °C. The endothermic peak on DTA curve with the minimum at 220 °C and the exothermic peak with the maxima at 396, 487, and 550 °C are visible. The main m/z values detected by mass spectrometer were 17 and 18 which can be ascribed to OH− and H2O molecules (Fig. 2b). This is therefore the main gaseous product released from the polymeric sample. The presence of CO2 is confirmed by m/z values 12 and 44. There is the increase in the intensities of MS 44 and 12 signals with the maxima at 240 and 550 °C what indicates on the decomposition of polymeric binder and oxidation of decomposition products (light hydrocarbons) to CO2. The stepped mass loss and the presence of a few maxima on MS curves indicate that thermal decomposition of the polymeric chain proceeds gradually. The MS signals 22, 24, 42, 45, 46, 55, 72 are observed with the maxima at 223 and 556 °C which can be ascribed to the first stage of thermal decomposition of the polymer (Fig. 2c). It must be underlined that the intensities of these signals are very low in comparison with signals 18 and 44. The major decomposition products come from main-chain scission, giving shorter polymeric chains (C1–C5 carbohydrates) like oligomers, trimers, dimers, and monomers [29, 30]. MS 45 peak of high intensity may indicate the carboxylic acid, ethoxy group, ethers, and alcohol groups, which correspond to the polymer structure [31, 32]. The presence of the m/z value 44 is observed till ca. 600 °C which means that mentioned above organic groups undergo further oxidation toward CO2.
Thermal degradation of SYNTRAN 8250, used as dispersing agent for alumina powder, is presented in Fig. 3a. The total mass loss was 98% what means that all of organic phase was decomposed. Mass loss starts at 133 °C and is observed until ca. 595 °C. The exothermic peak on DTA curve with maximum at 569 °C is visible. This peak overlaps with the peaks from mass spectrometer of m/z values 44, 22, 45, and 46 of the maximum at about 542 °C. The presence of masses 12 and 44 indicates the decomposition of the compound toward CO2. The highest intensities of MS signals 17 and 18 are observed in temperature range 200–590 °C which can be ascribed to H2O as one of the main products of SYNTRAN degradation (Fig. 3b). Mass spectrometer has detected also the following masses 24, 48, 60, and 64 with a maximum at 297 °C, 42 with a maximum at 432 °C and 22, 45, and 46 with a maximum at 546 °C. It can be therefore conducted that thermal decomposition of the polyacrylic acid homopolymer proceeds toward light hydrocarbons and followed by an oxidation of these products to CO2 (Fig. 3c). The decomposition products which come from main-chain scission, giving shorter hydrocarbons (C1–C5), are released in much smaller quantities in comparison with CO2 and H2O.
Comparing the thermal characteristics of polymer based on 2-carboxyethyl acrylate (Fig. 2) and SYNTRAN 8250 (Fig. 3), it can be concluded that the main stage of CEA polymer decomposition appears at lower temperatures (ca. 251 °C) than of SYNTRAN (ca. 504 °C). As a result, gases are released from the SYNTRAN sample at higher temperature.
The DTA/TG/DTG curves of thermal degradation of Al2O3 green body obtained by gelcasting with the use of the processing additives CEA and SYNTRAN 8250 are shown in Fig. 4a. The total mass loss was 5.55% which corresponds to the organic additives content in the analyzed sample. It means that all of the organic phase was burned out, as expected. Mass loss is observed until ca. 514 °C. Decomposition of organics goes in two stages according to TG curve. The endothermic peak on DTA curve with the minimum at 211 °C and the exothermic peak with the maximum at 385 °C are visible (Fig. 4b). The endothermic peak overlaps with the peaks from the mass spectrometer of m/z values 17 and 18 which can be ascribed to H2O, what indicates on dehydration process. The presence of CO2 is confirmed by m/z values 12 and 44. There is the increase in the intensities of MS 44 and 12 signals with the maximum at 375 °C, what indicates on the decomposition of the dispersing agent SYNTRAN and polymeric binder based on CEA toward light hydrocarbons, oligomers, trimers, dimmers followed by oxidation to CO2. The MS signals (22, 24, 42, 45, 46, 55, 72) detected during thermal treatment of Al2O3 green body (Fig. 4c) correspond to MS signals for analyzed polymer based on CEA and dispersant SYNTRAN. However, the MS peaks are much wider than in case of individual additives what means that in case of ceramic sample gases are released from the sample in a wide temperature range (100–550 °C).
The information gained from the above measurements is essential for suitable selection of sintering conditions of Al2O3 like sintering temperature and heating rate. It is very important because decomposition is connected with the release of high quantities of gases which may cause defects in the sample, and therefore it is preferable to conduct the sintering process with low heating rate, for example, 1 °C min−1 till the end of the organics decomposition. Sintering is the key step in the dense ceramic preparation process which should provide to obtain a high-quality product.
In the second part of the research, the ceramic–polymer tape based on BST and organic components were analyzed. The DTA/TG/DTG curves of thermal degradation of an ammonium salt of acrylic homopolymer in water (DURAMAX D-3005) which was used as dispersing agent for BST powder, are shown in Fig. 5a. The total mass loss was 98% what indicates that the whole DURAMAX D-3005 has decomposed. Mass loss starts at 362 °C and is observed until ca. 544 °C what means that DURAMAX D-3005 is much more thermally stable substance than SYNTRAN 8250 or polymer based on CEA. In case of BST ceramic–polymer composites it is very useful advantage. Two main stages of dispersant degradation are observed; nevertheless, 88% of mass loss is visible in the first stage till 397 °C. This first stage is connected with DTA exothermic peak with the maxima at 325 and 437 °C. The highest intensities of MS signals (of m/z values 12, 17, 18 and 44) are observed at temperature 386 °C (Fig. 5b). This can indicate partial decomposition of the compound toward H2O and CO2. The MS signals 22, 25, 42, 45, 55, 56 are observed at 373 °C which can be ascribed to thermal decomposition of the acrylic homopolymer toward CO2 (Fig. 5c). The main difference between DURAMAX D-3005 and the rest of the analyzed additives is that the decomposition of DURAMAX D-3005 proceeds rapidly, in the temperature range 363–397 °C, 88% of the substance is reduced.
DTA/TG/DTG curves of thermal degradation of an aqueous emulsion DURAMAX B-1000 used as a binder in the preparation of BST samples by tape casting, are shown in Fig. 6a. The total mass loss was 68% what could indicate that not whole DURAMAX B-1000 has decomposed; nevertheless, the mass loss is observed since the very beginning of the measurement, what can be ascribed to the sample volatility. The decomposition of DURAMAX B-1000 ends at ca. 498 °C which is 46 °C lower than in case of DURAMAX D-3005. Three stages of dispersant degradation are observed. The first stage till 102 °C is connected with the DTA endothermic peak at temperature 87 °C. The second and the third stages are connected with DTA exothermic peaks with the maxima at 430 and 670 °C. The first peak on MS curve of m/z values 17 and 18 is observed at temperature 53 °C (Fig. 6b). This can indicate dehydration process, but on the other hand confirms the hypothesis concerning high volatility of the sample. Mass spectrometer has detected also m/z values 12 and 44 having the highest intensities at 415 °C. The other MS signals 22, 42, 45, 55, and 56 are observed at 357 °C. Thus the situation is similar as in case of previous organic compounds, that is decomposition proceeds toward light carbohydrates which are then oxidized to CO2. The second main oxidation product is H2O. It is worth to underline that in case of DURAMAX B-1000 mass spectrometer has detected the smallest number of masses which means that thermal degradation proceeds with the release of smaller types of gaseous product (Fig. 6c).
DTA/TG/DTG curves of thermal degradation of the ceramic–polymer tape based on BST are shown in Fig. 7a. The total mass loss was 10% what corresponds to the content of the organic phase in the composite. Nevertheless, the concentration of dispersant and binder in a slurry was a little higher than the value of the total mass loss. It results from the fact that the mass of the sample taken to the analysis was ca. 0.3 g according to the capacity of the crucibles [18]. Mass loss starts at ca. 241 °C and is observed until ca. 448 °C. Tape decomposition goes in few stages according to TG curve. Three peaks are visible on DTA curve with maxima at 250, 383 and 410 °C. According to the information from mass spectrometer (Fig. 7b), course of curves of m/z values 17 and 18 is similar what indicates that they correspond to H2O. The presence of CO2 is confirmed by m/z values 12 and 44. There is the increase in the intensities of MS 44 and 12 signals with the maximum at 383 °C, which overlap with exothermic peak on DTA at 383 °C and indicates on the decomposition of organic phase toward CO2. The MS signals 22, 42, 45, 55, and 56 are observed at 342 °C (Fig. 7c) which can be ascribed to thermal decomposition of organic phase toward CO2. The most important information gained from this measurement is that the BST tape with the addition of the polymeric phase is thermally stable till 241 °C what is very beneficial from the application point of view. The BST tapes do not undergo the sintering process, because they are used as elastic ceramic–polymer materials exhibiting ferroelectric properties. Based on the literature review one can find thermal analysis of raw BST powder, but there was as yet no information about thermal stability of BST composites [27].
The microstructure of green body based on Al2O3 and ceramic–polymer composite based on BST is shown in Fig. 8. In SEM image of Al2O3 green body one can observe the polymer in the form of bridges which connect alumina particles (Fig. 8a). These polymeric bridges are responsible for high mechanical strength of Al2O3 sample before sintering. The particles in green body are highly densified. In BST composite a polymeric binder surrounds particles like a glue (Fig. 8b), what is responsible for elasticity of this composite tape. On the basis on SEM image, it can be estimated that the particles size was in the range of 0.3–1.2 µm. The polymeric phase visible in SEM images corresponds to the total mass loss estimated on the basis of TG curves of Al2O3 and BST samples.
Conclusions
Thermogravimetry coupled with mass spectrometry is a very useful tool to characterize the decomposition process of organic compounds as well as ceramic green bodies containing polymeric phase. Decomposition of the analyzed organics such as polymer based on 2-carboxyethyl acrylate or commercially available additives: SYNTRAN 8250, DURAMAX D-3005, and DURAMAX B-1000 proceeds toward light hydrocarbons with further oxidation to CO2. Rapid and fast degradation was observed for DURAMAX D-3005 which exhibited the highest thermal stability. In case of Al2O3 green body obtained by gelcasting, decomposition of organic additives ends at 514 °C, which is the important information in determination of the sintering program. BST tape is thermally stable till 241 °C which is very beneficial from the application point of view, because Ba0.8Sr0.2TiO3 together with the applied polymer exhibits ferroelectric properties and is used in a form of ceramic–polymer composite material.
References
Li J, Hastings GW. Oxide bioceramics: inert ceramic materials in medicine and dentistry. In: Murphy W, Black J, Hastings G, editors. Handbook of biomaterial properties. New York: Springer; 2016. p 339–352.
Ying S, Fuping W, Yu Z. Progresses of research on microwave dielectric ceramics. 1998; 02.
Hvizdos P, Puchy V, Duszova A, Dusza J, Balazsi C. Tribological and electrical properties of ceramic matrix composites with carbon nanotubes. Ceram Int. 2012;38:5669–76.
Kovalcikova A, Balazsi C, Dusza J, Tapaszto O. Mechanical properties and electrical conductivity in a carbon nanotube reinforced silicon nitride composite. Ceram Int. 2012;38:527–33.
Antosik A, Gluszek M, Zurowski R, Szafran M. Effect of SiO2 particle size and length of poly(propylene glycol) chain on rheological properties of shear thickening fluids. Arch Metall Mater. 2016;61:1165–8.
Tallon C, Franks GV. Recent trends in shape forming from colloidal processing: a review. J Ceram Soc Jpn. 2011;119:147–60.
Nieto MI, Suarez I, Moreno R. Shaping of dense advanced ceramics and coatings by gelation of polysaccharides. Adv Eng Mater. 2014;16:637–54.
Pietrzak E, Wiecinska P, Szafran M. 2-carboxyethyl acrylate as a new monomer preventing negative effect of oxygen inhibition in gelcasting of alumina. Ceram Int. 2016;42:13682–8.
Burgos-Montes O, Moreno R, Baudín C. Effect of mullite additions on the fracture mode of alumina. J Eur Ceram Soc. 2010;30:857–63.
Wiecinska P, Graule T, Bachonko M. Organic additives in gel-tape casting of ceramic powders—a novel approach to the problem of elasticity and cracking of thin tapes. J Eur Ceram Soc. 2015;35:3949–57.
Fan K, Ruiz-Hervias J, Gurauskis J, Sanchez-Herencia AJ, Baudín C. Neutron diffraction residual stress analysis of Al2O3/Y-TZP ceramic composites. Boletín de la Sociedad Española de Cerámica y Vidrio. 2016;55:13–23.
Takahashi M, Menchavez RL, Fuji M, Takegami H. Opportunities of porous ceramics fabricated by gelcasting in mitigating environmental issues. J Eur Ceram Soc. 2009;29:823–8.
Si W, Graule T, Baader F. Direct coagulation casting of silicon carbide components. J Am Ceram Soc. 1999;82:1129.
Schabikowski M, Zalewska M, Kata D, Graule T. The effect of CuO coatings on the electrokinetic properties of stone wool fibres determined by streaming potential measurements. Ceram Int. 2016;42:13944–51.
Bednarek P, Szafran M, Sakka Y, Mizerski T. Gelcasting of alumina with a new monomer synthesized from glucose. J Eur Ceram Soc. 2010;30:1795–801.
Szudarska A, Sakka Y, Suzuki TS, Mizerski T, Szafran M. Magnetic field alignment in highly concentrated suspensions for gelcasting process. Ceram Int. 2016;42:294.
Wiecinska P. Thermal degradation of organic additives used in colloidal shaping of ceramics investigated by the coupled DTA/TG/MS analysis. J Therm Anal Calorim. 2016;123:1419–30.
Bednarek P, Szafran M. Thermal decomposition of monosaccharides derivatives applied in ceramic gelcasting process investigated by the coupled DTA/TG/MS analysis. J Therm Anal Calorim. 2012;109:773–82.
Leszczynska A, Pielichowski K. Application of thermal analysis methods for characterization of polymer/montmorillonite nanocomposites. J Therm Anal Calorim. 2008;93:677–87.
Sczygiel I, Winiarska K. Synthesis and characterization of manganese–zinc ferrite obtained by thermal decomposition from organic precursors. J Therm Anal Calorim. 2014;115:471–7.
Golofit T, Zysk K. Thermal decomposition properties and compatibility of CL-20 with binders HTPB, PBAN, GAP and poly-NIMMO. J Therm Anal Calorim. 2015;119:1931–9.
Thom AJ, Summers E, Akinc M. Oxidation behavior of extruded Mo5Si3Bx–MoSi2–MoB intermetallics from 600 to 1600 °C. Intermetallics. 2002;10:555–70.
Etienne S, Becker C, Ruch D, Germain A, Calberg C. Synergetic effect of poly(vinyl butyral) and calcium carbonate on thermal stability of poly(vinyl chloride) nanocomposites investigated by TG–FTIR–MS. J Therm Anal Calorim. 2010;100:667–77.
Alcolea A, Ibarra I, Caparros A, Rodriguez R. Study of the MS response by TG–MS in an acid mine drainage efflorescence. J Therm Anal Calorim. 2010;101:1161–5.
Pietrzak E, Pawlikowska E, Godziszewski K, Yashchyshyn Y, Szafran M. Tunable ceramic–polymer composites for electronic applications. Compos Theory Pract. 2015;1:54–7.
Balachandran R, Ong BH, Wong HY, Tan KB, Muhamad Rasat M. Dielectric characteristics of barium strontium titanate based metal insulator metal capacitor for dynamic random access memory cell. Int J Electrochem Sci. 2012;7:11895–903.
Osinska K, Czekaj D. Thermal behavior of BST//PVDF ceramic–polymer composites. J Therm Anal Calorim. 2013;113:69–76.
Hu T, Juuti J, Jantunen H, Vilkman T. Dielectric properties of BST/polymer composite. J Eur Ceram Soc. 2007;27:3997–4001.
Bertini F, Audisio G, Zuev V. Investigation on thermal degradation of poly-n-alkyl acrylates and poly-n-alkyl methacrylates (C1–C12). Polym Degrad Stab. 2005;89:233–9.
Zuev VV, Bertini F, Audisio G. Investigation on the thermal degradation of acrylic polymers with fluorinated side-chains. Polym Degrad Stab. 2006;91:512–6.
Database of Mass Spectroscopy of Royal Society of Chemistry. www.rsc.org.
Database of Mass Spectroscopy of Kaye&Laby, National Physical Laboratory. www.kayelaby.npl.co.uk.
Acknowledgements
The project has been financially supported by National Science Centre, Poland (Agreement No. UMO-2014/15/D/ST5/02574). Authors would like to thank Interpolymer Company for free sample of SYNTRAN 8250, DOW for free samples of DURAMAX D-3005 and DURAMAX B-1000 and Almatis GmbH for free sample of Al2O3 A16SG which were used in the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pietrzak, E., Wiecinska, P., Pawlikowska, E. et al. Colloidal processing of Al2O3 and BST materials. J Therm Anal Calorim 130, 365–376 (2017). https://doi.org/10.1007/s10973-017-6401-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6401-6