Abstract
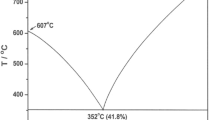
The phase equilibria in the terbium(III) chloride–lithium chloride pseudobinary system were established by means of differential scanning calorimetry. It was established that the pure terbium(III) chloride undergoes solid–solid phase transition at 790 K and melts at 859 K. The TbCl3–LiCl pseudobinary system is characterized by the existence of two compounds. First one, namely Li3TbCl6, forms at 553 K and melts incongruently at 727 K. Second compound, LiTbCl4, decomposes in the solid state at 609 K. The composition of Li3TbCl6–TbCl3 eutectic corresponding to terbium(III) chloride mole fraction x = 0.521 (T = 665 K) was found from Tammann plot, which predict, through application of the lever rule, the variation of the enthalpy associated with eutectic melting as a function of composition. The obtained results have been compared with the literature data concerning for the TbCl3–LiCl pseudobinary system. The phase diagram of the TbCl3–LiCl pseudobinary system was also optimized by CALPHAD method.








Similar content being viewed by others
References
Naumov VS, Bychkov AV, Lebedev A. Advenctes in molten salts: from structural aspects to waste processing. In: Gaude-Escard M, editor. Properties of liquid-salt nuclear fuel and its reprocessing technology. Danbury: Begell House Inc; 1999. p. 432–53.
Junming T, Bath NY. Quarty Metal Halide Lamps with Improved Lumen Maintenance. U.S. Patent Application Publication. 2008; US2008/0093993 Ai.
Junming T, Bath NY. Quarty Metal Halide Lamps with Improved Lumen Maintenance. U.S. Patent Application Publication. 2010; US 7, 786,674 B2.
Seifert HJ. Ternary chlorides of the trivalent late lanthanides: Phase diagrams, crystal structures and thermodynamic properties. J Therm Anal Calorim. 2006;83:479–505 and references therein.
Gmelin L. Handbook of inorganic chemistry, vol. C5. 8th ed. Berlin: Springer; 1977.
Prosypajko VI, Alekseeva EA. In: Bell HB, editor. Phase equilibria in binary halides. New York: IFI/Plenum; 1987.
Kutscher J, Schneider A. Zur Systematik der Zustandsdiagramme von Lanthaniden(III)-halogenid-Alkalihalogenid-System. Z Anorg Allg Chem. 1974;408:135–45.
Seifert HJ. Ternary chlorides of the trivalent early lanthanides: Phase diagrams, crystal structures and thermodynamic properties. J Therm Anal Calorim. 2002;67:789–826 and references therein.
Mitra S, Uebach J, Seifert HJ. Ternary chlorides in the systems ACl/TbCl3 (A = K, Rb, Cs). J Solid State Chem. 1995;115:484–9.
Seifert HJ, Sandrock J, Uebach J. Thermochemical and structural investigations on the systems NaCl/TbCl3 and NaCl/DyCl3. Acta Chem Scand. 1995;49:653–7.
Chao-Gui Z, Shou-Lin H, Si-Qiang W. Phase diagram of binary systems ErCl3–MCln (M = Li, Ca, Pb; n = 1 or 2). Chem J Chin Univ. 1993;14:992–5.
Chao-Gui Z, Shou-Lin H, Si-Qiang W. Phase diagram of binary systems YbCl3–MCln (M = Li, Mg, Ca, Pb; n = 1 or 2). Acta Phys Chim Sin. 1994;10:342–7.
Chao-Gui Z, Shou-Lin H, Si-Qiang W. Phase diagram of binary systems HoCl3–MCln (M = Li, Mg, Ca, Pb; n = 1 or 2). Acta Chim Sin. 1994;52:735–9.
Chao-Gui Z, Zhong-Dong Z, Si-Qiang W. Phase diagram of binary systems DyCl3–MCln (M = Li, Mg, Ca, Pb; n = 1 or n = 2). Inst Sci Tech Inf China. 1994;18:263.
Chao-Gui Z, Shou-Lin H, Si-Qiang W. Phase diagrams of the binary systems TbCl3-MCln (M = Li, Mg, Ca, Pb; n = 1 or 2). Trans Nonferrous Met Soc China. 1993;3(2):33.
Rycerz L, Gaune-Escard M. Enthalpies of phase transitions and heat capacity of TbCl3 and compounds formed in TbCl3–MCl systems (M = K, Rb, Cs). J Therm Anal Calorim. 2002;68:973–81.
Dworkin AS, Bredig MA. Enthalpy of lanthanide chloride, bromides and iodides from 298–1300 degrees k-enthalpies of fusion and transition. High Temp Sci. 1971;3(1):81.
Konings RJM, Kovacs A. Thermodynamic properties of the lanthanide(III) halides. Handb Phys Chem Rare Earths. 2003;33(213):147–247.
Goryushkin VF, Zalymova SA, Poshevneva AI. Thermal constants of the conversion of yttrium subgroup lanthanide trichloride. Zh Neorg Khim. 1990;35:3081.
Dańczak A, Rycerz L. Reinvestigation of the DyCl3–LiCl binary system phase diagram. J Therm Anal Calorim. 2016;126:299–305.
Kubaschewski O, Alcock CB, Spencer PJ. Materials thermochemistry. 6th ed. Oxford: Pergamon Press; 1998.
Findlay A. The phase rule and its applications. New York: Longmans, Green and Co.; 1911.
Guenet JM. Contributions of phase diagram to the understanding of organized polymer-solvent systems. Thermochim Acta. 1996;284:67–83.
Rycerz L. Practical remarks concerning phase diagram determination on the base of DSC measurements. J Therm Anal Calorim. 2013;113:231–8.
Lukas HL, Fries SG, Sundman B. Computational thermodynamics, the Calphad method. Cambridge: Cambridge University Press; 2007.
Saunders N, Miodownik AP. CALPHAD (calculation of phase diagrams): a comprehensive guide. 1st ed. Oxford: Pergamon; 1998.
Rycerz L, Gaune-Escard M. Mixing enthalpy of TbCl3–MCl liquid mixtures (M = Li, Na, K, Rb, Cs). High Temp Mater Proc. 1998;2(4):483–6.
Kapała J. Management program for BINGSS phase diagram optimizer. In: An international conference on phase diagram calculation and computational thermochemistry CALPHAD XXXIII, Kraków, 30.05–04.06.2004, 2004.
Lukas HL, Fries SG. Demonstration of the use of “BINGSS” with the Mg–Zn system as example. J Phase Equilib. 1992;13(5):532–41.
Szczygieł I, Salamon B, Kapała J. Modelling of thermodynamic properties of NdBr 3-MBr (M = Li–Cs) pseudobinary systems. CALPHAD. 2016;53:130–5.
Salamon B, Rycerz L, Kapała J, Gaune-Escard M. Phase diagram of NdI3–RbI pseudo-binary system. Thermodynamic properties of solid compounds. J Phase Equilib. 2015;404:9–16.
Kapała J, Salamon B. Thermodynamic assessment of TbCl3–MCl (M = Na, K, Rb, Cs) binary systems. CALPHAD. 2013;43:139–42.
Sommer F. Associacion model for the description of thermodynamic functions of liquid alloys part 1. Z Metallkd. 1982;73:72–6, and part 2. Z Metallkd. 1982;73:77–86.
Krull HG, Singh RN, Sommer F. Generalised association model. Z Metallkd. 2000;91:356–65.
Photiadis GM, Brresen B, Papatheodorou GN. Vibrational modes and structures of lanthanide halide-alkali halide binary melts LnBr 3-KBr (Ln = La, Nd, Gd) and NdCl3–ACl (A = Li, Na, K, Cs). J Chem Soc Farad Trans. 1998;94:2605–13.
Acknowledgements
The work was co-financed by a statutory activity subsidy from Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wrocław University of Science and Technology and for the Faculty of Engineering and Economics of Wrocław University of Economics. We would like to thank Dr. Hans Leo Lukas from Max-Planck-Institut für Metallforschung, Stuttgart, Germany, for his set of programs for optimization and calculation of the phase diagrams.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dańczak, A., Salamon, B., Kapała, J. et al. Reinvestigation of phase equilibria in TbCl3–LiCl binary system. J Therm Anal Calorim 130, 25–33 (2017). https://doi.org/10.1007/s10973-017-6315-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6315-3