Abstract
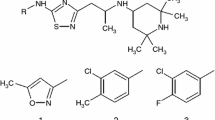
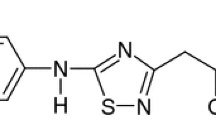
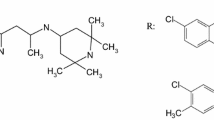
New 1,2,4-thiadiazole derivative displaying neuroprotective potential and high activity in the treatment of Alzheimer’s disease has been synthesized. The objective of this study was to improve the aqueous solubility of this drug-like compound by means of complex formation with native and hydroxypropylated β-cyclodextrins. To this end, aqueous solubility of 1,2,4-thiadiazole derivative was investigated in the presence of β-cyclodextrins. It was shown that the phase solubility diagrams are of Bs type demonstrating the initial increase in thiadiazole solubility in solutions of cyclodextrins (concentration up to 0.01 mol kg−1) followed by a solubility decrease due to the precipitation of the complexes formed. In comparison with β-cyclodextrin, hydroxypropyl-β-cyclodextrin displays more pronounced solubilizing action since it forms more stable complexes with thiadiazole. Solid complexes of 1,2,4-thiadiazole derivative with β-cyclodextrins were prepared by grinding and freeze-drying methods. DSC, TG, hot-stage microscopy, solid-state 13C MAS CP/TOSS NMR, powder X-ray diffractometry and FTIR spectroscopy were used to prove the existence of complexes in the solid state. Solubility of the obtained formulations was also examined. It was found that complexes of thiadiazole with hydroxypropyl-β-cyclodextrin exhibited higher solubility in phosphate buffer (pH 7.4) compared with pure thiadiazole and its complexes with β-cyclodextrin.









Similar content being viewed by others
References
Yihong Q, Yisheng C, Geoff GZZ, Lirong L, William P. Developing solid oral dosage forms: pharmaceutical theory and practice. New York: Academic; 2014.
Manin A, Voronin A, Drozd K, Simagina A, Churakov A, Perlovich G. Cocrystal screening of hydroxybenzamides with benzoic acid derivatives: a comparative study of thermal and solution-based methods. Eur J Pharm Sci. 2014;65:56–64.
Mishra A. Nanomedicine for drug delivery and therapeutics. London: Wiley; 2013.
Hadka P, Ro J, Kim H, Kim I, Kim J, Kim J, Kim H, Cho J, Yun G, Lee J. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 2014;9:304–16.
Brewster M, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–66.
Anand R, Manoli F, Manet I, Donzello M, Viola E, Malanga M, Jicsinszky L, Fenyvesi E, Monti S. Fluorescent cyclodextrin carriers for a water soluble Zn II pyrazinoporphyrazine octacation with photosensitizer potential. RCS Adv. 2014;4:26359–67.
Bouchal F, Skiba M, Chaffai N, Hallouard F, Fatmi S, Lahiana-Skida M. Fast dissolving cyclodextrin complex of piroxicam in solid dispersion Part I: influence of β-CD and HPβ-CD on the dissolution rate of piroxicam. Int J Pharm. 2015;478:625–32.
Mennini N, Bragagni M, Maestrelli F, Mura P. Physico-chemical characterization in solution and in the solid state of clonazepam complexes with native and chemically-modified cyclodextrins. J Pharm Biomed Anal. 2014;89:142–9.
Proshin A, Bachurin S. RF Pat. 2 449 997; 2012.
Volkova T, Terekhova I, Silyukov O, Proshin A, Bauer-Brandl A, Perlovich G. Towards the rational design of novel drugs based on solubility, partitioning/distribution, biomimetic permeability and biological activity exemplified by 1,2,4-thiadiazole derivatives. Chem Med Chem. 2016;. doi:10.1039/C6MD00545D.
Lin H, Lin S, Lin C, Hsu C, Wu T, Huang Y. Mechanical grinding effect on thermodynamics and inclusion efficiency of loratadine–cyclodextrin inclusion complex formation. Carbohydr Polym. 2012;87:512–7.
Jug M, Mennini N, Kover K, Mura P. Comparative analysis of binary and ternary cyclodextrin complexes with econazole nitrate in solution and in solid state. J Pharm Biomed Anal. 2014;91:81–91.
Higuchi T, Connors K. Advances in analytical chemistry and instrumentation. In: C.N. Reilly, editor. Vol. 4 New York: Wiley; 1965, pp. 212–117.
Chislov M, Silyukov O, Kumeev R, Proshin A, Perlovich G, Terekhova I. Complex formation of cyclodextrins with some pharmacologically active 1,2,4-thiadiazole derivatives. J Therm Anal Calorim. 2016;. doi:10.1007/s10973-016-5929-1.
Aytac Z, Kusku S, Durgun E, Uyar T. Quercetin/β-cyclodextrin inclusion complex embedded nanofibres: slow release and high solubility. Food Chem. 2016;197:864–71.
Ferreira M, João G, García A, Leonardi D, Salomon C, Lamas M, Nunes T. 13C and 15N solid-state NMR studies on albendazole and cyclodextrin albendazole complexes. Carbohydr Polym. 2015;123:130–5.
Ogawa N, Takahashi C, Yamamoto H. Physicochemical characterization of cyclodextrin–drug interactions in the solid state and the effect of water on these interactions. J Pharm Sci. 2015;104:942–54.
Locci E, Lai S, Piras A, Marongiu B, Lai A. 13C-CPMAS and 1H-NMR study of the inclusion complexes of beta-cyclodextrin with carvacrol, thymol, and eugenol prepared in supercritical carbon dioxide. Chem Biodivers. 2004;1:1354–66.
Lange K, Gierlach-Hładon T. Solid state characterization of α-tocopherol in inclusion complexes with cyclodextrins. Acta Pol Pharm. 2015;72:21–30.
Ford J. Current status of solid dispersions. J Pharm Acta Helv. 1986;61:69–88.
Periasamy R, Kothainayaki S, Sivakumar K. Investigation on inter molecular complexation between 4,4′-methylene-bis(N,N-dimethylaniline) and β-cyclodextrin: preparation and characterization in aqueous medium and solid state. J Mol Struct. 2015;1080:69–79.
Bikadi Z, Kurdi R, Bolqh S, Szeman J, Hazai E. Aggregation of cyclodextrins as an important factor to determine their complexation behavior. J Chem Biodivers. 2006;3:1266–78.
Gonzalez-Gaitano G, Rodriguez P, Isasi JR, Fuentes M, Tardajos G, Sanchez M. The aggregation of cyclodextrins as studied by photon correlation spectroscopy. J Incl Phenom Macrocycl Chem. 2002;44:101–5.
Messner M, Kurkov SV, Jansook P, Loftsson T. Self-assembled cyclodextrin aggregates and nanoparticles. Int J Pharm. 2010;387:199–208.
Acknowledgements
This work was supported by Russian Science Foundation (Grant No. 15-13-10017). Authors thank the “The upper Volga region Centre of physicochemical research” (Ivanovo, Russia) and St. Petersburg State University Research Park for the provision of scientific equipment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brusnikina, M., Silyukov, O., Chislov, M. et al. Effect of cyclodextrin complexation on solubility of novel anti-Alzheimer 1,2,4-thiadiazole derivative. J Therm Anal Calorim 130, 443–450 (2017). https://doi.org/10.1007/s10973-017-6252-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6252-1