Abstract
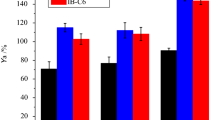
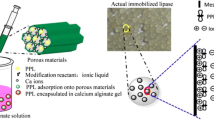
This work evaluated the capacity of protic ionic liquid (PIL) used as an additive to enhance the immobilization of lipase from Bacillus sp. in a sol–gel matrix. The immobilized derivatives were characterized with respect to the specific surface area, adsorption–desorption isothermic values, pore volume and size by nitrogen adsorption, thermal analysis and full recovery of activity. The lipases were immobilized using different PILs of the same cation (N-methylmonoethanolamine) and different anions (acetate, propionate, butyrate and pentanoate). The results showed that the total recovery of activity for the samples encapsulated in the presence of PIL was always higher than those without the encapsulated additive (total activity yield, Ya = 71%), particularly when using the more hydrophobic nature PILs (Ya = 305%), and at concentrations of 0.5 mass%. The positive effect of using PILs was also observed in the formation of the porous structure of the biocatalysts, as well as the increases in surface area (78 to 278 m2 g−1) and pore volume (0.018 to 0.414 cc g−1). The thermal analysis has revealed the key role of water content on the hydration shell of the enzyme caused by the change in the alkyl chain of ionic liquids, showing that the PILs are an excellent alternative for immobilization processes.




Similar content being viewed by others
References
Reetz MT, Tielmann P, Wiesenhofer W, Konen W, Zonta A. Second generation sol–gel encapsulated lipases: robust heterogeneous biocatalysts. Adv Synth Catal. 2003;345(6–7):717–28. doi:10.1002/adsc.200303016.
Reetz MT, Zonta A, Simpelkamp J. Efficient immobilization of lipases by entrapment in hydrophobic sol–gel materials. Biotechnol Bioeng. 1996;49(5):527–34.
Yagonia CFJ, Park K, Yoo YJ. Immobilization of Candida antarctica lipase B on the surface of modified sol–gel matrix. J Sol-Gel Sci Technol. 2014;69(3):564–70.
Zubiolo C, Santos RCA, Carvalho NB, Soares CMF, Lima AS, Santana LCLD. Encapsulation in a sol–gel matrix of lipase from Aspergillus niger obtained by bioconversion of a novel agricultural residue. Bioprocess Biosyst Eng. 2014;37(9):1781–8.
Souza RL, de Faria ELP, Figueiredo RT, Freitas LdS, Iglesias M, Mattedi S, et al. Protic ionic liquid as additive on lipase immobilization using silica sol–gel. Enzyme Microb Technol. 2013;52(3):141–50.
Binod P, Palkhiwala P, Gaikaiwari R, Nampoothiri KM, Duggal A, Dey K, et al. Industrial enzymes—present status and future perspectives for India. J Sci Ind Res India. 2013;72(5):271–86.
Kapoor M, Gupta MN. Lipase promiscuity and its biochemical applications. Process Biochem. 2012;47(4):555–69.
Jaeger K-E, Eggert T. Lipases for biotechnology. Curr Opin Biotechnol. 2002;13(4):390–7.
Sharma R, Chisti Y, Banerjee UC. Production, purification, characterization, and applications of lipases. Biotechnol Adv. 2001;19(8):627–62.
Carvalho NB, Silva MAD, Fricks AT, Franceschi E, Dariva C, Zanin GM, et al. Evaluation of activity of Bacillus lipase (free and immobilized) treated with compressed propane. J Mol Catal B-Enzym. 2014;99:130–5.
Cabrera-Padilla RY, Albuquerque M, Figueiredo RT, Fricks AT, Franceschi E, Lima AS, et al. Immobilization and characterisation of a lipase from a new source, Bacillus sp ITP-001. Bioprocess Biosyst Eng. 2013;36(10):1385–94.
Kumar D, Parshad R, Gupta VK. Application of a statistically enhanced, novel, organic solvent stable lipase from Bacillus safensis DVL-43. Int J Biol Macromol. 2014;66:97–107.
Barbosa JMP, Souza RL, de Melo CM, Fricks AT, Soares CMF, Lima AS. Biochemical characterisation of lipase from a new strain of Bacillus sp ITP-001. Quim Nova. 2012;35(6):1173–8.
Guncheva M, Zhiryakova D. Catalytic properties and potential applications of Bacillus lipases. J Mol Catal B-Enzym. 2011;68(1):1–21.
Huang XJ, Yu AG, Xu ZK. Covalent immobilization of lipase from Candida rugosa onto poly(acrylonitrile-co-2-hydroxyethyl methacrylate) electrospun fibrous membranes for potential bioreactor application. Bioresour Technol. 2008;99(13):5459–65.
Souza R, Faria EP, Figueiredo R, Fricks A, Zanin G, Santos OA, et al. Use of polyethylene glycol in the process of sol–gel encapsulation of Burkholderia cepacia lipase. J Therm Anal Calorim. 2014;117:301–6.
Lee SH, Doan TTN, Ha SH, Chang W-J, Koo Y-M. Influence of ionic liquids as additives on sol–gel immobilized lipase. J Mol Catal B-Enzym. 2007;47(3–4):129–34.
Karout A, Pierre AC. Silica xerogels and aerogels synthesized with ionic liquids. J Non-Cryst Solids. 2007;353(30–31):2900–9.
Hu Y, Tang SS, Jiang L, Zou B, Yang J, Huang H. Immobilization of Burkholderia cepacia lipase on functionalized ionic liquids modified mesoporous silica SBA-15. Process Biochem. 2012;47(12):2291–9.
Hara P, Mikkola JP, Murzin DY, Kanerva LT. Supported ionic liquids in Burkholderia cepacia lipase-catalyzed asymmetric acylation. J Mol Catal B-Enzym. 2010;67(1–2):129–34.
Zarcula C, Corici L, Croitoru R, Ursoiu A, Peter F. Preparation and properties of xerogels obtained by ionic liquid incorporation during the immobilization of lipase by the sol-gel method. J Mol Catal B-Enzym. 2010;65(1–4):79–86.
Zou B, Song C, Xu X, Xia J, Huo S, Cui F. Enhancing stabilities of lipase by enzyme aggregate coating immobilized onto ionic liquid modified mesoporous materials. Appl Surf Sci. 2014;311:62–7.
Carvalho NB, Lima AS, Soares CMF. Use of modified silicas for lipase immobilization. Quim Nova. 2015;38(3):399–409.
Thuy Pham TP, Cho C-W, Yun Y-S. Environmental fate and toxicity of ionic liquids: a review. Water Res. 2010;44(2):352–72.
Ventura SPM, Gonçalves AMM, Sintra T, Pereira JL, Gonçalves F, Coutinho JAP. Designing ionic liquids: the chemical structure role in the toxicity. Ecotoxicology. 2013;22(1):1–12.
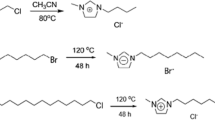
Alvarez VH, Dosil N, Gonzalez-Cabaleiro R, Mattedi S, Martin-Pastor M, Iglesias M, et al. Bronsted ionic liquids for sustainable processes: synthesis and physical properties. J Chem Eng Data. 2010;55(2):625–32.
Maximo GJ, Santos RJBN, Lopes-da-Silva JA, Costa MC, Meirelles AJA, Coutinho JAP. Lipidic protic ionic liquid crystals. ACS Sustain Chem Eng. 2014;2(4):672–82.
Alvarez VH, Mattedi S, Martin-Pastor M, Aznar M, Iglesias M. Synthesis and thermophysical properties of two new protic long-chain ionic liquids with the oleate anion. Fluid Phase Equilib. 2010;299(1):42–50.
Greaves TL, Drummond CJ. Protic ionic liquids: properties and applications. Chem Rev. 2008;108(1):206–37.
Barbosa JMP, Souza RL, Fricks AT, Zanin GM, Soares CMF, Lima AS. Purification of lipase produced by a new source of Bacillus in submerged fermentation using an aqueous two-phase system. J Chromatogr B. 2011;879(32):3853–8.
INPI, Patent submission No. PI0306829-3, 11 Sept 2003.
Soares CMF, De Castro HF, De Moraes FF, Zanin GM. Characterization and utilization of Candida rugosa lipase immobilized on controlled pore silica. Appl Biochem Biotechnol. 1999;77–9:745–57.
Souza RL, Resende WC, Barao CE, Zanin GM, de Castro HF, Santos OAA, et al. Influence of the use of Aliquat 336 in the immobilization procedure in sol-gel of lipase from Bacillus sp ITP-001. J Mol Catal B-Enzym. 2012;84:152–9.
Zhou Y. Recent advances in ionic liquids for synthesis of inorganic nanomaterials. Curr Nanosci. 2005;1(1):35–42.
Vila-Real H, Alfaia AJ, Rosa JN, Gois PMP, Rosa ME, Calado ART, et al. alpha-Rhamnosidase and beta-glucosidase expressed by naringinase immobilized on new ionic liquid sol–gel matrices: activity and stability studies. J Biotechnol. 2011;152(4):147–58.
Paul C, Borza P, Marcu A, Rusu G, Bîrdeanu M, Zarcula SM, et al. Influence of the physico-chemical characteristics of the hybrid matrix on the catalytic properties of sol–gel entrapped Pseudomonas fluorescens lipase. Nanomater Nanotechnol. 2016;. doi:10.5772/62194.
Soares CMF, dos Santos OA, de Castro HF, de Moraes FF, Zanin GM. Characterization of sol–gel encapsulated lipase using tetraethoxysilane as precursor. J Mol Catal B-Enzym. 2006;39(1–4):69–76.
Wei Y, Xu JG, Dong H, Dong JH, Qiu KY, Jansen-Varnum SA. Preparation and physisorption characterization of d-glucose-templated mesoporous silica sol–gel materials. Chem Mater. 1999;11(8):2023–9.
Mukherjee I, Mylonakis A, Guo Y, Samuel SP, Li SX, Wei RY, et al. Effect of nonsurfactant template content on the particle size and surface area of monodisperse mesoporous silica nanospheres. Micropor Mesopor Mater. 2009;122(1–3):168–74.
Acknowledgements
The authors acknowledge the financial support of FAPITEC/SE, CAPES and CNPq (Process 23038-028317/2008-44).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souza, R.L., Faria, E.L.P., Figueiredo, R.T. et al. Protic ionic liquid applied to enhance the immobilization of lipase in sol–gel matrices. J Therm Anal Calorim 128, 833–840 (2017). https://doi.org/10.1007/s10973-016-5950-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5950-4