Abstract
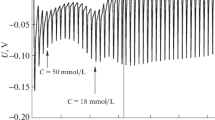
This paper reports experimental results regarding the solubility of argon and nitrogen in aqueous solutions of tetradecyltrimethylammonium bromide (TTAB) at temperatures between 283.15 and 298.15 K, 101,325 Pa partial pressure of gas, and 0.04–0.20 mol kg−1 of TTAB concentration. Measurements were taken in specially developed equipment, following the change in gas pressure. The gas solubility was determined applying Henry’s law. The experimental results show that the solubility of argon and nitrogen in the TTAB micelles is 62.5–86.3 times higher than the solubility in pure water. The solubility of the studied gases is larger in the TTAB micelles than in the DTAB determined in a previous work under the same experimental conditions, showing the effect of the addition of a methylene group in the alkyl chain of DTAB. Comparing the results obtained, it can be observed that the TTAB micelles are larger and have a higher solubilization capacity.


Similar content being viewed by others
References
Battino R, Clever HL. The solubility of gases in liquids. Chem Rev. 1966;66:395–463.
Battino R, Seybold PG. The O2/N2 ratio gas solubility mystery. J Chem Eng Data. 2011;56:5036–44.
Raposo RR, Calviño E, Esteso MA. A new electrochemical method for the determination of gas solubility in aqueous solutions. J Electroanal Chem. 2008;617:157–63.
Battino R, Seybold PG, Campanell FC. Correlations involving the solubility of gases in water at 298.15 K and 101325 Pa. J Chem Eng Data. 2011;56:727–32.
Hefter GT, Tomkins RPT, editors. The experimental determination of solubilities, vol. 6. 1st ed. West Sussex: Wiley; 2003.
Davie MK, Zatsepina OY, Buffett BA. Ethane solubility in marine hydrate environments. Mar Geol. 2004;203:177–84.
Yalkowsky SH. Solubility and solubilization in aqueous media. 1st ed. New York: Oxford University Press; 1999.
Laurence S, editor. Surfactant science and technology: retrospects and prospects. 1st ed. Boca Ratón: CRC Press; 2014.
Adamson AW. Physical chemistry of surfaces. 6th ed. New York: Wiley; 1997.
Roy S, Mehra A, Bhowmick D. Prediction of solubility of nonpolar gases in micellar solutions of ionic surfactants. J Colloid Interface Sci. 1997;196:53–61.
Calhoun AR, King AD Jr. The solubility of ethane in aqueous solutions of sodium 1-pentanesulfonate, sodium 1-hexanesulfonate, sodium 1-heptanesulfonate, and sodium 1-octanesulfonate at 25 °C. J Colloid Interface Sci. 2007;309:505–10.
Rosen MJ. Surfactants and interfacial phenomena. 3rd ed. Hoboken: Wiley; 2004.
Blandamer MJ, Cullis PM, Soldi LG, Engberts JBFN, Kacperska A, Van Os NM, Subha MCS. Thermodynamics of micellar systems: comparison of mass action and phase equilibrium models for the calculation of standard Gibbs energies of micelle formation. Adv Colloid Interface Sci. 1995;58:171–209.
Cui X, Mao S, Liu M, Yuan H, Du Y. Mechanism of surfactant micelle formation. Langmuir. 2008;24:10771–5.
Ben-Naim A, Wilf J. Solubility and thermodynamics of solution of argon in aqueous solutions of sodium octanoate and sodium dodecylsulfate. J Solut Chem. 1983;12:861–8.
Ben-Naim A, Battino R. Solubilization of methane, ethane, propane and n-butane in aqueous solutions of sodium dodecylsulfate. J Solut Chem. 1985;14:245–53.
Prapaitrakul W, King AD Jr. The solubility of gases in aqueous solutions of decyltrimethyl- and cetyltrimethylammonium bromide. J Colloid Interface Sci. 1985;106:186–93.
Serra MCC, Coelho JPA, Calado JCG, Palavra AMF. Solubility of argon in micellar aqueous solutions of sodium dodecyl sulfate. J Colloid Interface Sci. 1995;173:278–83.
King AD Jr. The solubility of ethane, propane, and carbon dioxide in aqueous solutions of sodium cumene sulfonate. J Colloid Interface Sci. 2004;273:313–9.
Mirgorod YA. Solubility of ethane, propane, and butane in aqueous solutions of sodium dodecyl sulfate. Russ J Gen Chem. 2005;75:31–3.
Romero CM, Garzon LC, Blanco LH, Suarez AF. Solubility of argon and nitrogen in aqueous solutions of dodecyltrimethylammonium bromide (DTAB) from 283.15 to 298.15 K and 101325 Pa partial pressure of gas. J Solut Chem. 2014;43:1147–55.
Mosquera V, del Rıo JM, Attwoodb D, Garcıa M, Jones MN, Prieto G, Suarez J, Sarmiento FA. Study of the aggregation behavior of hexyltrimethylammonium bromide in aqueous solution. J Colloid Interface Sci. 1998;206:66–76.
Evans DF, Allen M, Ninham BW, Fouda A. Critical micelle concentrations for alkyltrimethylammonium bromides in water from 25 to 160 °C. J Solut Chem. 1984;13:87–101.
Kudryashov E, Kapustina T, Morrissey S, Buckin V, Dawson K. The compressibility of alkyltrimethylammonium bromide micelles. J Colloid Interface Sci. 1998;203:59–68.
Thermophysical Properties of Fluid Systems. In: National Institute of Standards and Technology (NIST). http://webbook.nist.gov/chemistry/fluid. Accessed 17 Oct 2015.
Berry RS, Rice SA, Ross J. Physical chemistry. 3rd ed. New York: Oxford University Press; 2000.
De Lisi RD, Milioto S, Verrall RE. Partial molar volumes and compressibilities of alkyltrimethylammonium bromides. J Solut Chem. 1990;19:665–92.
Silva WP, Silva CMDPS. LAB Fit Curve Fitting Software (Nonlinear Regression and Treatment of Data Program) V 7.2.48 (1999–2011). http://www.labfit.net. Accessed 5 Dec 2015.
Acknowledgements
The authors would like to thank Professor Luis H. Blanco (1945–2012) for his advice and support in the development of this work. Professor Blanco was a faculty member of the Department of Chemistry from Universidad Nacional de Colombia. This work was supported by Universidad Nacional de Colombia, Universidad de Bogotá Jorge Tadeo Lozano and Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología, Francisco José de Caldas - COLCIENCIAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garzon, L.C.A., Suarez, A.F. & Romero, C.M. Solubility of argon and nitrogen in aqueous solutions of tetradecyltrimethylammonium bromide from 283.15 to 298.15 K and 101,325 Pa partial pressure of gas. J Therm Anal Calorim 128, 475–479 (2017). https://doi.org/10.1007/s10973-016-5888-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5888-6