Abstract
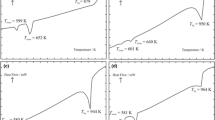
Phase diagram of HoBr3–KBr pseudo-binary system was established by differential scanning calorimetry (DSC) method. This system includes two intermediate compounds: K3HoBr6 and K2HoBr5. K3HoBr6 undergoes a solid–solid phase transition at 689 K and melts congruently at 992 K, while K2HoBr5 melts incongruently at 736 K. In the HoBr3–KBr, system two eutectics occur. The eutectic mixture e1 melts at 875 K; its composition is x(HoBr3) = 0.162. The other eutectic e2 with composition of x(HoBr3) = 0.455 melts at 699 K. The heat capacity (solid and liquid phase) of congruently melting compound formed in this system was measured by DSC in the 300–1273 K range. The specific conductivity of HoBr3–KBr liquid mixtures was measured down to temperatures below solidification over the entire composition range. The obtained results are discussed in term of possible complexes formation.








Similar content being viewed by others
References
Markus T, Ohnesorge M, Mollenhoff M, Hilpert K. Aspects of high temperature chemistry in metal halide lamps. In: Taxil P, Bessada C, Cassir M, Gaune-Escard M, editors. Proceedings of the 7th international symposium on molten salts chemistry and technology. Toulouse, France, August 29 to September 2, 2005. p. 743–6.
Mucklejohn SA, Dinsdale AT. The behaviour of metal halide systems in high intensity discharge lamps. In: Taxil P, Bessada C, Cassir M, Gaune-Escard M, editors. Proceedings of the 7th international symposium on molten salts chemistry and technology. Toulouse, France, August 29 to September 2, 2005. p. 7761–4.
van Erk W. Transport processes in metal halide discharge lamps. Pure Appl Chem. 2000;72:2159–66.
Guest EC, Mucklejohn SA, Preston B, Rouffet JB, Zissis G. “NumeLiTe”: an energy effective lighting system for roadways and an industrial application of molten salts. In: Oye HA, Jagtoyen A, Carry le Rouet, editors. Proceedings of international symposium on ionic liquids in honour of M. Gaune-Escard; France, June 26–28, 2003. p. 37–45.
Mucklejohn SA, Trindell D, Devonshire R. An assesment of the thermochemical parameters for the lanthanide triiodides. Presented as poster paper 37P on: 6th international symposium science & technol. Light Sources, Budapest, Hungary, 30 August–3 September 1992.
Brumleve TR, Voggenauer K, Lovell ME, Scott TE, DeHaven D.B, Gordon D, Steward RL, Hansen SC. Spectra of rare-earth iodide-cesium iodide systems in low-wattage metal halide lamp. In: Proceedings of the 11th international symposium on the science and technology of light sources, Shanghai, China, May 20–24, 2007; Paper CP090.
Kirby MW. Mercury lamps. In: Coaton JR, Marsden AM, editors. Lamps and lighting, 4th ed. London; 1997. p. 227–62.
Preston B, Odell EC, Metal halide lamps. In: Coaton JR, Marsden AM, editors. Lamps and lighting, 4th ed. London; 1997. p. 263–80.
Mucklejohn SA. Molten salt high-intensity discharge lamps. In: Proceedings of the international george papatheodorou symposium, Patras, September 17–18, 1999. pp. 63–7.
US Patent 3761758 A.
US Patent 4686419 A.
US Patent 7319294 B2.
Hilpert K, Niemann U. High temperature chemistry in metal halide lamps. Thermochim Acta. 1997;299:49–57.
Markus T, Niemann U, Hilpert K. High temperature gas phase chemistry for the development of advanced ceramic discharge lamps. J Phys Chem Solids. 2005;66:372–5.
Mucklejohn SA. Standard enthalpies of formation, entropies and heat capacities of the lanthanide monohalides, LnX < g> X = F, Cl, Br, I. J Phys D Appl Phys. 2011;44(22):224010.
Cybińska J, Legendziewicz J, Boulon G, Bensalah A, Meyer G. Assignment of spectroscopic properties in praseodymium-doped and praseodymium/ytterbium-co-doped ternary K2LaX5 (X = Cl, Br, I) Halides. Opt Mater. 2006;28:41–52.
Hawrami R, Batra AK, Aggarwal MD, Roy UN, Groza M, Cui Y, Burger A, Cherepy N, Niedermayr T, Payne SA. New scintillator materials (K2CeBr 5 and Cs2CeBr5). J Cryst Growth. 2008;310:2099–102.
Ingole DK, Joshi CP, Moharil SV, Muthal PL, Dhopte SM. Wet-chemical preparation of Ce3+-activated K2LaX5 (X = Cl, Br or I) phosphors. Luminescence. 2012;27(1):24–7.
Brown D, Fletcher S, Holah DG. Preparation and crystallographic properties of certain lanthanide and actinide tribromides and tribromide hexahydrates. J Chem Soc (A). 1968;8:1889–94.
Dudareva AG, Polanskaja OW, Tenanko NW, Ezhov AI, Strekachinskii AB. Interaction of holmium tribromide with alkali metal bromides. Zh Neorg Khim. 1984;29:2646–50.
Jantsch G, Wein K. Uber die Schmelzpunkte, insbesondere der Bromide. Monatsch. Chem. 1939;69:161–6.
Gmeli L. Gmelin Handuch der Anorganischen Chemie, Sc, Y, La und lanthanide, system-Nr 39, Teil C6. Berlin: Springer; 1978. p. 22–3.
Meyer G, Dotsch S, Staffel T. The ammonium bromide route to anhydrous rare earth bromides MBr 3. J Less Comm Met. 1987;127:155–60.
Rycerz L, Chojnacka I, Berkani M, Gaune-Escard M. Thermodynamic functions of PrBr 3 and congruently melting M3PrBr6 compounds (M = K, Rb, Cs). J Chem Eng Data. 2011;56:1293–8.
Fouque Y, Gaune-Escard M, Szczepaniak W, Bogacz A. Synthesis, electrical conductibility and phase transition entropy measurements of the Na2UBr 6 compound. J Chim Phys. 1978;75:360–6.
Janz GJ. Molten salts data as reference standards for density, surface tension, viscosity and electrical conductance: KNO3 and NaCl. J Phys Chem Ref Data. 1980;9:791–829.
Dworkin AS, Breding MA. Enthalpy of lanthanide chlorides, bromides and iodides from 298 to 1300 K: enthalpies of fusion and transition. High Temp. Sci. 1971;3:81–90.
Rycerz L, Ingier-Stocka E, Gaune-Escard M. Phase diagram and electrical conductivity of the CeBr 3-CsBr binary system. J Therm Anal Calorim. 2009;97:1015–21.
Rycerz L, Ingier-Stocka E, Gadzuric S, Gaune-Escard M. Electrical conductivity of the molten EuBr 2–MBr binary mixtures (M = Li, Na, K, Rb, or Cs). J Mol Liq. 2004;140:78–83.
Markov BF, Shumina LA. The concentration dependence of the electroconductivity of binary salt melts. Zh Fiz Khim. 1957;31:1767–73.
Delimarskii YK. Elektrokhimija ionnych rasplavov. Metallurgija: Moskva; 1978. p. 34–6.
Wojakowska A, Plinska S, Josiak J. Electrical conductivity of molten cobalt dichloride-potassium chloride mixtures In: Gaune-Escard M, editors. Advances in molten salts: from structural aspects to waste processing, proceedings of the European research conference on molten salts. Porquerolles, France, 1998. p. 676–82.
Photiadis GM, Borresen B, Papatheodorou GN. Vibrational modes and structures of lanthanide halide–alkali halide binary melts LnBr 3–KBr (Ln = La, Nd, Gd) and NdCl3–ACl (A = Li, Na, K, Cs). J Chem Soc Faraday Trans. 1998;17:2605–13.
Potapov A, Gaune-Escard M. Deviations from the Arrhenius equation of electrical conductivity polytherms. In: Øye HA, Jagtøyen A, editors. Proccedings of the international symposium on ionic liquids in honour of professor Marcelle Gaune-Escard. Carry le Rouet, France; 2003, June 26–28. p. 477–81.
Acknowledgements
The work was co-financed by statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Engineering and Economics of Wrocław University of Economics and for the Faculty of Chemistry of Wroclaw University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pilarek, B., Rycerz, L. & Szczygieł, I. Thermodynamic and transport properties of the HoBr3–KBr pseudo-binary system. J Therm Anal Calorim 125, 1125–1133 (2016). https://doi.org/10.1007/s10973-016-5496-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5496-5