Abstract
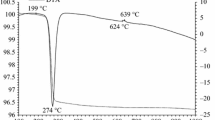
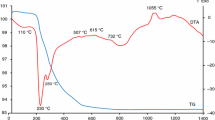
This research is focused on the synthesis, characterization and optical properties of novel environmentally friendly inorganic pigment Bi2Ce2O7. Powder sample was prepared by the conventional ceramic method, i.e. solid-state reaction. Prepared pigments were applied into organic matrix in mass tone and ceramic glaze. Thermal behaviour of the reaction mixture was investigated using differential thermal and thermogravimetric analyses. The optimum conditions for synthesis of the compound were determined. The pigment Bi2Ce2O7 was evaluated from standpoint of its structure, colour and particle sizes. The phase composition of the product was studied by X-ray diffraction analysis. Colourimetric parameters were evaluated by measuring the reflectance in the visible region of light. The resulting colour hue of studied pigment in an organic binder is yellow to yellow orange, depending on the calcination temperature. Characterization of Bi2Ce2O7 pigment suggests that it has a potential to be alternative yellow or yellow-orange colourant for paint, plastics, ceramics and building materials.


Similar content being viewed by others
References
Buxbaum G, Pfaff G. Industrial inorganic pigments. 3rd ed. Weinheim: Wiley; 2005.
Rosi F, Manuali V, Miliani C, Brunetti BG, Sgamellotti A, Grygar T, Hradil D. Raman scattering features of lead pyroantimonate compounds. Part I: XRD and Raman characterization of Pb2Sb2O7 doped with tin and zinc. J Raman Spectrosc. 2009;40:107–11.
Šulcová P. Thermal stability and colour properties of new pigments based on BiREO3. J Therm Anal Calorim. 2012;109:639–42.
Subramanian MA, Aravamudan G, Rao GVS. Oxide pyrochlores—a review. Prog Solid State Chem. 1983;15:55–143.
Harwig HA, Gerards AG. Electrical-properties of alpha, beta, gamma and delta phases of bismuth sesquioxide. J Solid State Chem. 1978;26:265–74.
Takahashi T, Iwahara H. Oxide ion conductors based on bismuthsesquioxide. Mater Res Bull. 1978;13:1447–53.
Drache M, Roussel P, Wignacourt JP. Structures and oxide mobility in Bi–Ln–O materials: heritage of Bi2O3. Chem Rev. 2007;107(1):80–96.
Mehring M. From molecules to bismuth oxide-based materials: potential homo- and heterometallic precursors and model compounds. Coord Chem Rev. 2007;251(7–8):974–1006.
Thompson M, Herranz T, Santos B, Marco JF, Berry FJ, Greaves C. The ionic conductivity and local environment of cations in Bi9ReO17. J Solid State Chem. 2010;183:1985–91.
Jiang N, Wachsman ED, Jung S-H. A higher conductivity Bi2O3-based electrolyte. Solid State Ionics. 2002;150:347–53.
Iwahara H, Esaka T, Sato T, Takahashi T. Formation of high oxide ion conductive phases in the sintered oxides of the system Bi2O3–Ln 2O3 (Ln = La –Yb). J Solid State Chem. 1981;39:173–80.
Belver C, Adán C, Fernández-García M. Photocatalytic behaviour of Bi2MO6 polymetalates for rhodamine B degradation. Catal Today. 2009;143:274–81.
Saha D, Madras G, Row TNG. Synthesis and structure of Bi2Ce2O7: a new compound exhibiting high solar photocatalytic activity. Dalton Trans. 2012;41:9598–600.
Ho C-H, Chan C-H, Huang Y-S, Tien L-C, Chao L-C. The study of optical band edge property of bismuth oxide nanowires α-Bi2O3. Opt Soc Am. 2013;21:1–8.
Zhou Q, Blanchard PER, Kennedy BJ, Ling ChD, Liu S, Avdeev M, Aitken JB, Tadich A, Brand HEA. Diffraction and spectroscopic study of pyrochlores Bi2−xFe1+xSbO7. J Alloys Compd. 2014;589:425–30.
Wuensch BJ, Eberman KW. Order–disorder phenomena in A2B2O7 pyrochlore oxides. JOM. 2000;52(7):19–21.
Pirzada M, Grimes RW, Minervini L, Maguire JF, Sickafus KE. Oxygen migration in A2B2O7 pyrochlores. Solid State Ion. 2001;140:201–8.
Hradil D, Grygar T, Hradilová J, Bezdička P, Grűnwaldová V, Fogaš I, Miliani C. Microanalytical identification of Pb–Sb–Sn yellow pigment in historical European paintings and its differentiation from lead tin and Naples yellows. J Cult Herit. 2007;8:377–86.
CPMA. Classification and chemical descriptions of the complex inorganic color pigments. 4th ed. Alexandria, VA: Color Pigments Manufacturers Association, Inc.; 2010.
Stránská L, Šulcová P, Vlček M. Synthesis and properties of inorganic pigments based on pyrochlore compounds with different lanthanides. J Therm Anal Calorim. 2013;113:127–35.
Völz HG. Industrial color testing: fundamentals and techniques. 2nd ed. Weinheim: Wiley; 2002.
Šulcová P, Trojan M. Thermal synthesis and properties of the (Bi2O3)1−x (Ho2O3) x pigments. J Therm Anal Calorim. 2006;83:557–9.
Šulcová P, Večeřa J, Strnadlová L. Study of doped CeO2 prepared by different synthesis. J Therm Anal Calorim. 2012;108:519–23.
Brisse F, Knop O. Pyrochlores. II. An investigation of La2Ce2O7 by neutron diffraction. Can J Chem. 1967;45(6):609–14.
Joint Committee on Powder Diffraction Standards. International centre of diffraction data. Swarthmore, PA, USA; 2012.
Acknowledgements
The authors would like to thank the IGA University of Pardubice (SGSFChT_2015005) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Těšitelová, K., Šulcová, P. Synthesis and study of Bi2Ce2O7 as inorganic pigment. J Therm Anal Calorim 125, 1047–1052 (2016). https://doi.org/10.1007/s10973-016-5322-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5322-0