Abstract
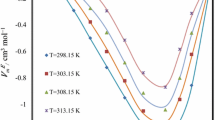
In the present work, new data on the densities and refractive indices for 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP] with N-methyldiethanolamine are reported for various concentrations and at temperatures (303.15–328.15) K. Excess molar volumes \({V^{\text{E}}}\) and excess refractive indices \( n_{\text{D}}^{\text{E}}\) were calculated from the experimental data. Refractive index values for the binary mixtures were predicted by using Lorentz–Lorenz, Gladstone–Dale and Eykman equations. Excess molar volumes showed positive trend, whereas excess refractive indices showed negative trend over the entire range of temperatures and concentrations studied in the present research.



Similar content being viewed by others
References
Han X, Armstrong DW. Ionic liquids in separations. Acc Chem Res. 2007;40:1079–86.
Navarro P, Larriba M, Beigbeder JB, Garcia J, Rodriguez F. Thermal stability and specific heats of [bpy][BF4] + [bpy][Tf2 N] and [bpy][BF4] + [4bmpy][Tf2 N] mixed ionic liquid solvents. J Therm Anal Calorim. 2015;119:1235–43.
Chen Y, Zhang S, Yuan X, Zhang Y, Zhang X, Dai W, Mori R. Solubility of CO2 in imidazolium-based tetrafluoroborate ionic liquids. Thermochim Acta. 2006;441:42–4.
Usula M, Plechkova NV, Piras A, Porcedda S. Ethylammonium alkanoate-based ionic liquid + water mixtures. A calorimetric and volumetric study at 298.15 K. J Therm Anal Calorim. 2015;. doi:10.1007/s10973-015-4753-3.
Ziyada AK, Bustam MA, Wilfred CD, Murugesan T. Densities, viscosities, and refractive indices of 1-hexyl-3-propanenitrile Imidazolium ionic liquids incorporated with sulfonate-based anions. J Chem Eng Data. 2011;56:2343–8.
Bukalak D, Kuceba IM, Nowak W. Assessment of the sorption capacity and regeneration of carbon dioxide sorbents using thermogravimetric method. J Therm Anal Calorim. 2013;113:157–60.
Cadena C, Anthony JL, Shah JK, Morrow TI, Brennecke JF, Maginn EJ. Why is CO2 So soluble in imidazolium-based ionic liquids? J Am Chem Soc. 2004;126:5300–8.
Aki SNVK, Mellein BR, Saurer EM, Brennecke JF. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J Phys Chem B. 2004;108:20355–65.
Yuan X, Zhang S, Liu J, Lu X. Solubilities of CO2 in hydroxyl ammonium ionic liquids at elevated pressures. Fluid Phase Equilib. 2007;257:195–200.
Tang J, Sun W, Tang H, Radosz M, Shen Y. Enhanced CO2 absorption of poly(ionic liquid)s. Macromolecules. 2005;38:2037–9.
Ahmady A, Hashim MA, Aroua MK. Absorption of carbon dioxide in the aqueous mixtures of methyldiethanolamine with three types of imidazolium-based ionic liquids. Fluid Phase Equilib. 2011;309:76–82.
Sairi NA, Yusoff R, Alias Y, Aroua MK. Solubilities of CO2 in aqueous N-methyldiethanolamine and guanidinium trifluoromethanesulfonate ionic liquid systems at elevated pressures. Fluid Phase Equilib. 2011;300:89–94.
Camper D, Bara JE, Gin DL, Noble RD. Room-temperature ionic liquid—amine solutions: tunable solvents for efficient and reversible capture of CO2. Ind Eng Chem Res. 2008;47:8496–8.
Feng Z, Gang FC, Ting WY, Tao WY, Min LA, Bing ZZ. Absorption of CO2 in the aqueous solutions of functionalized ionic liquids and MDEA. Chem Eng J. 2010;160:691–7.
Rho SW, Yoo KP, Lee JS, Nam SC, Son JE, Min BM. Solubility of CO2 in aqueous methyldiethanolamine solutions. J Chem Eng Data. 1997;42:1161–4.
Taib MM, Murugesan T. Densities and excess molar volumes of binary mixtures of bis(2-hydroxyethyl)ammonium acetate + water and monoethanolamine + Bis(2-hydroxyethyl)ammonium acetate at temperatures from (303.15 to 353.15) K. J Chem Eng Data. 2010;55:5910–3.
Taib MM, Ziyada AK, Wilfred CD, Murugesan T. Thermophysical properties of 1-propyronitrile-3-hexylimidazolium bromide + methanol at temperatures (293.15 to 323.15) K. J Mol Liq. 2011;158:101–4.
Akbar MM, Murugesan T. Thermophysical properties of 1-hexyl-3-methylimidazolium tetrafluoroborate [hmim][BF4] + N-methyldiethanolamine (MDEA) at temperatures (303.15 to 323.15) K. J Mol Liq. 2013;177:54–9.
Alghawas HA, Hagewiesche DP, Ibanez GR, Sandall OC. Physiochemical properties important for carbon dioxide absorption in aqueous methyldiethanolamine. J Chem Eng Data. 1989;34:385–91.
Garcia JMB, Estrada MR, Silva GAI, Hall KR. Densities and excess molar volumes of aqueous solutions of n-methyldiethanolamine (MDEA) at temperatures from (283.15 to 363.15) K. J Chem Eng Data. 2003;48:1442–5.
Alvarez E, Cerdeira F, Gomez-Diaz D, Navaza JM. Density, speed of sound, isentropic compressibility, and excess volume of (monoethanolamine + 2-amino-2-methyl-1-propanol), (monoethanolamine + triethanolamine), and (monoethanolamine + N-methyldiethanolamine) at temperatures from (293.15 to 323.15) K. J Chem Eng Data. 2010;55:994–9.
Muhammad A, Mutalib MIA, Wilfred CD, Murugesan T, Shafeeq A. Viscosity, refractive index, surface tension, and thermal decomposition of aqueous N-Methyldiethanolamine solutions from (298.15 to 338.15) K. J Chem Eng Data. 2008;53:2226–9.
Souckova M, Klomfar J, Patek J. Temperature dependence of the surface tension and 0.1 MPa density for 1-C n -3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate with n = 2, 4, and 6. J Chem Thermodyn. 2012;48:267–75.
Kondaiah M, Sreekanth K, Kumar DS, Nayeem SM, Rao DK. Densities, viscosities, and excess properties for binary mixtures of ethylene glycol with amides at 308.15 K. J Therm Anal Calorim. 2014;118:475–83.
Sankar MG, Ponneri V, Kumar KS, Sakamuri S. Molecular interactions between amine and cyclic ketones at different temperatures. J Therm Anal Calorim. 1821;115:1821–7.
Vural US, Muradoglu V, Vural S. Excess molar volumes, and refractive index of binary mixtures of glycerol + methanol and glycerol + water at 198.15 K and 303.15 K. Bull Chem Soc Ethiop. 2011;25(1):111–8.
Sudake Y, Kamble SP, Tidar AL, Maharolkar AP, Patil SS, Khirade PW. Study of intermolecular interaction of Allyl chloride with 2-pentanone and 2-hexanone through excess molar volume and excess molar refraction. J Pharm Biol Chem Sci. 2011;2:761–70.
Akbar MM, Murugesan T. Thermophysical properties for the binary mixtures of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [hmim][Tf2N] + N-methyldiethanolamine (MDEA) at temperatures (303.15 to 323.15)K. J Mol Liq. 2012;169(169):95–101.
Sovilj M, Barjaktarovic B. Excess molar volumes of ternary liquid systems containing aliphatic alcohols at several temperatures. Bull Chem Technol Maced. 2000;19:73–8.
Reis JCR, Lampreia IMS, Santos AFS, Moita MLCJ, Douheret G. Refractive index of liquid mixtures: theory and experiment. ChemPhysChem. 2010;11:3722–33.
Acknowledgements
The financial support by MyRA-Malaysia incentive grant through CO2 Rich Natural Gas Value Chain Program is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akbar, M.M., Chemat, F., Arunagiri, A. et al. Density and excess properties of N-methyldiethanolamine (MDEA) with 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP]. J Therm Anal Calorim 123, 785–791 (2016). https://doi.org/10.1007/s10973-015-4957-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4957-6