Abstract
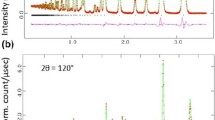
Struvite (MgNH4PO4·6H2O) was heated to temperatures from 55 to 300 °C. X-ray diffraction analysis revealed struvite was stable at 55 °C, partially decomposed to dittmarite (MgNH4PO4·H2O) at 100–200 °C, and formed an amorphous phase at 250–300 °C. Thermogravimetric analysis confirmed sample mass loss consistent with dittmarite formation at 100–200 °C and evolution of all volatiles at 250–300 °C. Fourier transform infrared (FTIR) spectroscopy detected the ν4 NH +4 band in 55–200 °C solids, as expected for struvite and dittmarite. This band decreased in intensity at 250 °C, and was not evident at 300 °C, confirming loss of NH +4 (s) at these temperatures. FTIR spectra also showed changes in the vibrations of the ν3 PO 3−4 band. At 55 °C, splitting in the band indicated destabilization of the PO 3−4 (s) group despite no change in mineralogy. Vibrations at 100–200 °C were associated with dittmarite and MgHPO4, and at 250–300 °C, MgHPO4 and Mg2P2O7. Analysis of acid-digested solids indicated the presence of P other than P–PO4 at 200–250 °C, confirming Mg2P2O7 formation. Overall, heat treatment of struvite produces several decomposition products, complete identification of which requires the use of multiple approaches. Temperature-induced phase transformations along with emission of NH3(g) have implications for use of struvite in multiple applications.




Similar content being viewed by others
References
Cordell D, Drangert JO, White S. The story of phosphorus: global food security and food for thought. Glob Environ Change. 2009;19(2):292–305.
Bezbaruah AN, Almeebi T. Nanoparticle-sorbed phosphate: iron and phosphate bioavailability studies with Spinacia oleracea and Selenastrum capricornutum. ACS Sustain Chem Eng. 2014;2(7):1625–32.
Paerl HW, Huisman J. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Rep. 2009;1(1):27–37.
Smith VH, Tilman DG, Nekola JC. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut. 1991;100(1):179–96.
Liu YH, Kwag JH, Kim JH, Ra CS. Recovery of nitrogen and phosphorus by struvite crystallization from swine wastewater. Desalination. 2011;277(1):364–9.
Bhuiyan MIH, Mavinic DS, Koch FA. Thermal decomposition of struvite and its phase transition. Chemosphere. 2008;70(8):1347–56.
Neyens E, Baeyens J. A review of thermal sludge pre-treatment processes to improve dewaterability. J Hazard Mater. 2003;98(1):51–67.
Rouff AA. The use of TG/DSC–FT-IR to assess the effect of Cr sorption on struvite stability and composition. J Therm Anal Calorim. 2012;110(3):1217–23.
Rouff AA. Temperature-dependent phosphorus precipitation and chromium removal from struvite-saturated solutions. J Colloid Interface Sci. 2013;392:343–8.
Mestres G, Ginebra MP. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011;7(4):1853–61.
Mestres G, Aguilera FS, Manzanares N, Sauro S, Osorio R, Toledano M, Ginebra MP. Magnesium phosphate cements for endodontic applications with improved long-term sealing ability. Int Endod J. 2014;47(2):127–39.
Michałowski T, Pietrzyk A. A thermodynamic study of struvite + water system. Talanta. 2006;68(3):594–601.
Sarkar AK. Hydration/dehydration characteristics of struvite and dittmarite pertaining to magnesium ammonium phosphate cement systems. J Mater Sci. 1991;26(9):2514–8.
Frost RL, Weier ML, Erickson KL. Thermal decomposition of struvite. J Therm Anal Calorim. 2004;76(3):1025–33.
Whitaker A. The decomposition of struvite. Mineral Mag. 1968;36:820–4.
Kurtulus G, Tas AC. Transformations of neat and heated struvite (MgNH4PO4·6H2O). Mater Lett. 2011;65(19):2883–6.
Abdelrazig BEI, Sharp JH. Phase changes on heating ammonium magnesium phosphate hydrates. Thermochim Acta. 1988;129(2):197–215.
Paul I, Varghese G, Ittayachen MA. Thermal decomposition studies of struvites. Indian J Pure Appl Sci. 2002;40(9):664–9.
Šoptrajanov B, Stefov V, Kuzmanovski I, Jovanovski G, Lutz HD, Engelen B. Very low H–O–H bending frequencies. IV. Fourier transform infrared spectra of synthetic dittmarite. J Mol Struct. 2002;613(1):7–14.
Rouff AA, Juarez KM. Zinc interaction with struvite during and after mineral formation. Environ Sci Technol. 2014;48(11):6342–9.
Boonchom B. Kinetic and thermodynamic studies of MgHPO4·3H2O by non-isothermal decomposition data. J Therm Anal Calorim. 2009;98(3):863–71.
Frost RL, Weier ML, Martens WN, Henry DA, Mills SJ. Raman spectroscopy of newberyite, hannayite and struvite. Spectrochim Acta A Mol Biomol Spectrosc. 2005;62A:181–8.
Umbreit MH, Paukszta D. The influence of temperature (20–1000 °C) on binary mixtures of solid solutions of CH3COOLi·2H2O–MgHPO4·3H2O. Phys B Condens Matter. 2009;404(20):3620–36.
Ma N, Rouff AA, Phillips BL. A 31P NMR and TG/DSC–FTIR Investigation of the influence of initial pH on phosphorus recovery as struvite. ACS Sustain Chem Eng. 2014;2(4):816–22.
Jeong YK, Kim JS. A new method for conservation of nitrogen in aerobic composting processes. Bioresour Technol. 2001;79:129–33.
Yetilmezsoy K, Sapci-Zengin Z. Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure wastewater by MAP precipitation as a slow release fertilizer. J Hazard Mater. 2009;166(1):260–9.
Vorndran E, Ewald A, Müller FA, Zorn K, Kufner A, Gbureck U. Formation and properties of magnesium–ammonium–phosphate hexahydrate biocements in the Ca–Mg–PO4 system. J Mater Sci Mater Med. 2011;22(3):429–36.
Moseke C, Saratsis V, Gbureck U. Injectability and mechanical properties of magnesium phosphate cements. J Mater Sci Mater Med. 2011;22(12):2591–8.
Chau CK, Qiao F, Li Z. Microstructure of magnesium potassium phosphate cement. Constr Build Mater. 2011;25(6):2911–7.
Zhang S, Shi HS, Huang SW, Zhang P. Dehydration characteristics of struvite-K pertaining to magnesium potassium phosphate cement system in non-isothermal condition. J Therm Anal Calorim. 2013;111(1):35–40.
Acknowledgements
Support was provided by the National Science Foundation Grant No. EAR-1506653. The authors thank Ning Ma for assistance with sample preparation and analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramlogan, M.V., Rouff, A.A. An investigation of the thermal behavior of magnesium ammonium phosphate hexahydrate. J Therm Anal Calorim 123, 145–152 (2016). https://doi.org/10.1007/s10973-015-4860-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4860-1