Abstract
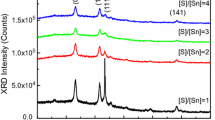
Thermal decomposition of precursors for SnS thin films obtained by drying aqueous solutions of SnCl2 and SC(NH2)2 in the Sn:S molar ratios of 1:1 (1) and 1:8 (2) was monitored by simultaneous thermogravimetry/differential thermal analysis (TG/DTA) measurements in the dynamic 80 % Ar + 20 % O2 atmosphere. The evolved gaseous species from 1 were recorded by simultaneous thermogravimetry/differential thermal analysis/evolved gas analysis–mass spectrometry (TG/DTA/EGA-MS) measurements. X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) were used to characterize the dried precursors and products of the thermal decomposition. The precursor 1 is a complex compound, Sn(tu)Cl2, while 2 consists of Sn2(tu)5Cl4·H2O and noncomplexed thiourea. The thermal degradation of 1 and 2 in the temperature range of 30–700 °C consists of four steps with a total mass loss of 68.0 and 84.5 %, respectively. According to XRD, the solid decomposition products at 210 °C of 1 are SnS2, (NH4)2SnCl6 and C(NH2)3SnCl3, and of 2 are SnS2, SnS, NH4Cl and (NH4)2SnCl6. Decomposition of 1 and 2 is completed by 550 and 570 °C, respectively. The final decomposition product of 1 and 2 at 700 °C is SnO2. This study shows that the excess of thiourea in the spray solution, compared to that required for the formation of the intermediate tin complex compounds, suppresses the formation of tin oxide phase and depresses the loss of tin species from the system.





Similar content being viewed by others
References
Sinsermsuksakul P, Hartman K, Kim SB, Heo J, Sun L, Park HH, Chakraborty R, Buonassisi T, Gordon RG. Enhancing the efficiency of SnS solar cells via band-offset engineering with a zinc oxysulfide buffer layer. Appl Phys Lett. 2013;102:053901.
Reddy KTR, Reddy PP, Miles RW, Datta PK. Investigation of SnS films deposited by spray pyrolysis. Opt Mater. 2001;17:295–8.
Jeyaprakash BG, Kumar RA, Kesavan K, Amalarani A. Structural and optical characterization of spray deposited SnS thin film. J Am Sci. 2010;6:22–6.
Krunks M, Kärber E, Katerski A, Otto K, Oja Acik I, Dedova T, Mere A. Extremely thin absorber layer solar cells on zinc oxide nanorods by chemical spray. Sol Energy Mater Sol Cells. 2010;94:1191–5.
Otto K, Katerski A, Mere A, Volobujeva O, Krunks M. Spray pyrolysis deposition of indium sulphide thin films. Thin Solid Films. 2011;519:3055–60.
Dedova T, Krunks M, Gromyko I, Mikli V, Sildos I, Utt K, Unt T. Effect of Zn: S molar ratio in solution on the properties of ZnS thin films and the formation of ZnS nanorods by spray pyrolysis. Phys Status Solidi A. 2014;2:514–21.
Sajeesh TH, Warrier AR, Kartha CS, Vijayakumar KP. Optimization of parameters of chemical spray pyrolysis technique to get n and p-type layers of SnS. Thin Solid Films. 2010;518:4370–4.
Ray SC, Karanjai MK, DasGupta D. Structure and photoconductive properties of dip-deposited SnS and SnS2 thin films and their conversion to tin dioxide by annealing in air. Thin Solid Films. 1999;350:72–8.
Polivtseva S, Oja Acik I, Katerski A, Mere A, Mikli V, Krunks M. Spray pyrolysis deposition of SnxSy thin films. Energy Procedia. 2014;60:156–65.
Madarász J, Bombicz P, Okuya M, Kaneko S. Thermal decomposition of thiourea complexes of Cu(I), Zn(II), and Sn(II) chlorides as precursors for the spray pyrolysis deposition of sulfide thin films. Solid State Ionics. 2001;141–142:439–46.
Madarász J, Bombicz P, Okuya M, Kaneko S, Pokol G. Comparative online coupled TG-FTIR and TG/DTA-MS analyses of the evolved gases from thiourea complexes of SnCl2: tetrachloropenta(thiourea) ditin(II), a compound rich in thiourea. J Anal Appl Pyrolysis. 2004;72:209–14.
Madarász J, Bombicz P, Okuya M, Kaneko S, Pokol G. Online coupled TG–FTIR and TG/DTA–MS analyses of the evolved gases from dichloro(thiourea) tin(II). Solid State Ion. 2004;172:577–81.
NIST Chemistry WebBook Standard Reference Database No 69, June 2005 Release, http://webbook.nist.gov/chemistry.
Krunks M, Madarász J, Hiltunen L, Mannonen R, Mellikov E, Niinistö L. Structure and thermal behaviour of dichlorobis(thiourea)cadmium(II), a single-source precursor for CdS thin films. Acta Chem Scand. 1997;51:294–301.
El-Bahy GMS, El-Sayed BA, Shabana AA. Vibrational and electronic studies on some metal thiourea complexes. Vib Spectrosc. 2003;31:101–7.
Otto K, Oja Acik I, Tõnsuaadu K, Mere A, Krunks M. Thermoanalytical study of precursors for In2S3 thin films deposited by spray pyrolysis. J Therm Anal Calorim. 2011;105:615–23.
Bombicz P, Mutikainen I, Krunks M, Leskelä T, Madarász J, Niinistö L. Synthesis, vibrational spectra and X-ray structures of copper (I) thiourea complexes. Inorg Chim Acta. 2004;357:513–25.
Krunks M, Mellikov E, Bijakina O. Intermediate compounds in formation of copper sulfides by spray pyrolysis. Proc Estonian Acad Sci Engin. 1996;2:98–106.
International Centre for Diffraction Data (ICDD), Powder Diffraction File (PDF), PDF-2 Release 2008.
Donaldson JD, Grimes SM. X-Ray crystal structure determinations of two thiourea tin(II) complexes: diacetatobis(thiourea)tin(II) and ditin(II)tetrabromopenta(thiourea)dihydrate. Inorg Chim Acta. 1984;84:173–7.
Cassidy JE, Moser W, Donaldson JD, Jelen A, Nicholson DG (1970) Thiourea complexes of tin(II) compounds. J Chem Soc A, 173–175.
Oja Acik I, Otto K, Krunks M, Tõnsuaadu K, Mere A. Thermal behaviour of precursors for CuInS2 thin films deposited by spray pyrolysis. J Therm Anal Calorim. 2013;113:1455–65.
Madarász J, Pokol G. Comparative evolved gas analysis on thermal degradation of thiourea by coupled TG-FTIR and TG/DTA-MS instruments. J Therm Anal Calorim. 2007;88:329–36.
Krunks M, Leskelä T, Mannonen R, Niinistö L. Thermal decomposition of copper(I) thiocarbamide chloride hemihydrate. J Therm Anal Calorim. 1998;53:355–64.
Otto K, Bombicz P, Madarász J, Oja Acik I, Krunks M, Pokol G. Structure and evolved gas analyses (TG/DTA-MS and TG-FTIR) of mer-trichlorotris(thiourea)-indium(III), a precursor for indium sulfide thin films. J Therm Anal Calorim. 2011;105:83–91.
Krunks M, Madarász J, Leskelä T, Mere A, Niinistö L, Pokol G. Study of zinc thiocarbamide chloride, a single-source precursor for zinc sulfide thin films by spray pyrolysis. J Therm Anal Calorim. 2003;72:497–506.
Nyquist RA, Putzig CL, Leugers MA. Handbook of infrared and Raman spectra of inorganic compounds and organic salts: text and explanations, vol. 1. San Diego: Academic Press; 1997.
Krunks M, Leskelä T, Mutikainen I, Niinistö L. A thermoanalytical study of copper(I) thiocarbamide compounds. J Therm Anal Calorim. 1999;56:479–84.
Wang S, Gao Q, Wang J. Thermodynamic analysis of decomposition of thiourea and thiourea oxides. J Phys Chem B. 2005;109:17281–9.
Ristic M, Ivanda M, Popovic S, Music S. Dependence of nanocrystalline SnO2 particle size on synthesis route. J Non-Cryst Solids. 2002;303:270–80.
Acknowledgements
This study was financially supported by the Estonian Ministry of Education and Research (IUT-19-4), the Estonian Science Foundation (ETF9081), and by the European Union through the European Regional Development Fund (Projects: 3.2.0101.11-0029 and 3.2.1101.12-0023). The authors thank Dr. Valdek Mikli for EDS analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polivtseva, S., Oja Acik, I., Krunks, M. et al. Thermoanalytical study of precursors for tin sulfide thin films deposited by chemical spray pyrolysis. J Therm Anal Calorim 121, 177–185 (2015). https://doi.org/10.1007/s10973-015-4580-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4580-6