Abstract
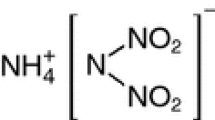
Basic copper nitrate [Cu(NO3)2·3Cu(OH)2, BCN] is a widely used oxidizer for gas-generating compounds. The oxidizers that replace some BCN with ammonium nitrate (NH4NO3, AN) have been investigated to increase the performance of the gas-generating agents. The purpose of this study was to understand the thermal behavior and stability of AN/BCN mixtures. To this end, mixtures prepared by two kinds of methods, with and without heat treatment, were analyzed by X-ray powder diffraction to investigate composition of samples, and differential scanning calorimetry and thermogravimetry–differential thermal analysis with mass spectrometry (TG–DTA–MS) to investigate the thermal behavior and evolved gases. It was found that [Cu(NH3)2](NO3)2 was formed in the sample with thermal treatment. The samples with and without heating exhibited different decomposition processes. It is considered that the residual AN and the amount of [Cu(NH3)2](NO3)2 in the mixture affected the decomposition behavior.






Similar content being viewed by others
References
Mei X, Cheng Y, Li Y, Zhu X, Yan S, Li X. Thermal decomposition properties of guanidine nitrate and basic curip nitrate. J Therm Anal Calorim. 2013;111:131–5.
Bucerius KM, Schmid HM. US Patent 5,542,999; 1996.
Barnes MW, Taylor RD, Hock C. US Patent 5,635,668; 1997.
Barnes MW, Taylor RD. US Patent 5,608,183. 1997;03:04.
Taylor RD, Mendenhall IV. Burn rate enhancement of basic copper nitrate-containing gas generant compositions: U. S. Patent 7,998,292 B2. 2011;08:16.
L’vov BV, Novichikhin AV. Mechanism of thermal decomposition of hydrated copper nitrate in vacuo. Spectrochim Acta B. 1995;50:1459–68.
Ghose J, Kanungo A. Studies on the thermal decomposition of Cu(NO3)2·3H2O. J Therm Anal. 1981;20:459–62.
Badica P, Aldica G, Crisan A. Decomposition of Ca:Cu = 1:1 nitrate powder. Thermal analysis and structural studies. J Mater Sci. 2002;37:585–94.
Ryu SK, Lee WK, Park SJ. Thermal decomposition of hydrated copper nitrate [Cu(NO3)2·3H2O] on activated carbon fibers. Carbon Sci. 2004;5:180–5.
Ilcheva L, Maneva M, Bozadziev P. Thermal investigation of the basic copper (II) nitrate Cu(OH)1.5(NO3)0.5. J Therm Anal. 1979;16:205–7.
Bottaro JC, Penwell PE, Schmitt RJ. 1,1,3,3-tetraoxo-1,2,3-triazapropene anion, a new oxy anion of nitrogen: the dinitramide anion and its salts. J Am Chem Soc. 1997;119:9405–10.
Pak Z. Some ways to higher environmental safety of solid rocket propellant application. In: Proc AIAA/SAE/ASME/ASEE 29th joint propulsion conf exhibition. Monterey; 1993.
Östmark H, Bemm U, Langlet A, Sanden R, Wingborg N. The properties of ammonium dinitramide (ADN): part 1, basic properties and spectroscopic data. J Energ Mater. 2000;18:123–8.
Oommen C, Jain SR. Ammonium nitrate: a promising rocket propellant oxidizer. J Hazard Mater. 1999;67:253–81.
Oxley JC, Smith JL, Rogers E, Yu M. Ammonium nitrate: thermal stability and explosivity modifiers. Thermochim Acta. 2002;384:23–45.
Oxley JC, Smith JL, Zheng W, Rogers E, Coburn MD. Thermal decomposition studies on ammonium dinitramide (ADN) and 15N and 2H isotopomers. J Phys Chem A. 1997;101:5642–52.
Sinditskii VP, Egorshev Y, Levshenkov AI, Serushkin VV. Combustion of ammonium dinitramide, part 1: burning behavior. J Propul Power. 2006;22:769–76.
Matsunaga H, Yoshino S, Kumasaki M, Habu H, Miyake A. Aging characteristics of the energetic oxidizer ammonium dinitramide. Sci Tech Energ Mater. 2011;72:131–5.
Matsunaga H, Habu H, Miyake A. Influences of aging on thermal decomposition mechanism of high performance oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;113:1387–94.
Matsunaga H, Habu H, Miyake A. Thermal behavior of new oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;111:1183–8.
Matsunaga H, Habu H, Miyake A. Thermal decomposition of the high-performance oxidizer ammonium dinitramide under pressure. J Therm Anal Calorim. 2014;116:1227–32.
Fujisato K, Habu H, Hori K. Condensed phase behavior in the combustion of ammonium dinitramide. Propellant Explos Pyrotech. 2014;39:714–22.
Fujisato K, Habu H, Hori K. Role of additives in the combustion of ammonium dinitramide. Propellant Explos Pyrotech. 2014;39:518–22.
Sugie Y, Miyake A. Effects of temperature on nitration of sulfamates. J Therm Anal Calorim. 2014;116:1213–7.
Sinditskii VP, Egorshev VY, Levshenkov AI, Serushkin VV. Ammonium nitrate: combustion mechanism and the role of additives. Propellant Explos Pyrotech. 2005;30:269–80.
Wada Y, Arai M. A study on ammonium nitrate-metal nitrate double salts as oxidizers for gas generating agent. Sci Tech Energ Mater. 2010;71:39–43.
Miyata Y, Hasue K. Burning characteristics of aminoguanidinium 5,5′-Azobis- 1H tetrazolate/ammonium nitrate mixture-effects of particle size and composition ratio on burning rate. J Energ Mater. 2011;29:344–59.
Kohga M, Okamoto K. Thermal decomposition behaviors and burning characteristics of ammonium nitrate/polytetrahydrofuran/glycerin composite propellant. Combust Flame. 2011;158(578–82):15.
Nakamura H, Saeki K, Akiyoshi M, Takahasi K. The reaction of ammonium nitrate with carbon powder. Kayaku Gakkaishi. 2002;63:87–93 (In Japanese).
Pandey M, Jha S, Kumar R, Mishra S, Jha RR. The pressure effect study on the burning rate of ammonium nitrate-HTPB-based propellant with the influence catalysts. J Therm Anal Calorim. 2012;107:135–40.
Golovina N, Nechiporenko G. Ammonium nitrate phase state stabilization with small amounts of some organic compounds. Cent Eur J Energ Mater. 2009;6:45–56.
Golovina N, Nechiporenko G. Phase state stabilization of ammonium nitrate for creating an oxidizing agent for smokeless gas-generating formulations yielding no toxic combustion products. Russ J Appl Chem. 2007;80:24–30.
Vorozhtsov A, Archipov V, Bondarchuk S, Popok N, Klyakin G, Babuk V, Luca LTD, Galfetti L. Ballistic characteristics of solid propellants containing dual oxidizer. In: Proc 1st Europ Conf. Aerospace Sci Moscow; 2005.
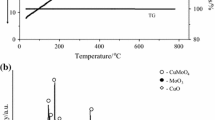
Sudhakar AOR. Mathew S. Thermal behavior of CuO doped phase-stabilised ammonium nitrate. Thermochim Acta. 2006;451:5–9.
Kajiyama K, Izato Y, Miyake A. Thermal characterristics of ammonium nitrate, carbon, and copper (II) oxide mixtures. J Therm Anal Calorim. 2013;113:1475–85.
Izato Y, Miyake A, Date S. Combustion characteristics of ammonium nitrate and carbon mixtures based on a thermal decomposition mechanism. Propellant Explos Pyrotech. 2013;38:129–35.
Izato Y, Kajiyama K, Miyake A. Thermal decomposition mechanism of ammonium nitrate and copper(II) oxide mixtures. Sci Tech Energ Mater. 2014;75:128–33.
Nagayama S, Katoh K, Higashi E, Nakano K, Kumagae K, Habu H, Wada Y, Arai M. Differential scanning calorimetry analysis of crystal structure transformation in spray-dried particles consisting of ammonium nitrate, potassium nitrate, and a polymer. J Therm Anal Calorim. 2014;118:1215–19.
Nagayama S, Katoh K, Higashi E, Nakano K, Habu H. Effect of polymer addition amount and type on thermal decomposition behavior of spray-dried particles comprising ammonium nitrate, potassium nitrate, and polymer. J Therm Anal Calorim. 2014;118:1221–27.
Wada Y, Hori K, Arai M. Combustion mechanism of mixtures of guanidine nitrate, ammonium nitrate, and basic copper nitrate. Sci Technol Energ Mater. 2010;71:83–7.
Dyukarev SS, Morozov IV, Reshetova LN, Guz OV, Arkhangel’skii IV, Korenve YM, Spiridonov FM. Copper(II) nitrate ammoniates Cu(NH3)4(NO3)2 and Cu(NH3)2(NO3)2 and their thermolysis under reduced pressure. Russ J Inorg Chem. 1999;44:883–8.
Southern TM, Wendlandt WW. The thermal decomposition of metal complexes—XX: some amine copper(II) nitrate complexes. J Inorg Nucl Chem. 1970;32:3783–92.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiota, K., Matsunaga, H. & Miyake, A. Thermal analysis of ammonium nitrate and basic copper(II) nitrate mixtures. J Therm Anal Calorim 121, 281–286 (2015). https://doi.org/10.1007/s10973-015-4536-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4536-x