Abstract
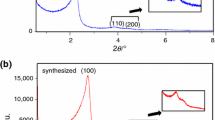
Thermogravimetry analysis was used to study the thermal decomposition of Ti-MCM-41 to determine the best calcination conditions. Ti-MCM-41 molecular sieve was synthesized using a hydrothermal route in which Ti ions were incorporated into the pore channels of MCM-41 starting from a hydrogel method according to the following molar composition: 1.00 CTMABr:4.00 SiO2:X TiO2:1.00 Na2O:200.00 H2O. Physicochemical properties of samples were investigated using XRD, FTIR, and N2 adsorption–desorption to confirm the incorporation of TiO2 nanoparticles inside of MCM-41 mesoporous without destroying its structure. The high temperatures facilitate the rapid removal of the surfactant; however, it caused destruction of the MCM-41 structure by breaking the bonds of the silica tetrahedral structure. The procedures to obtain the apparent activation energies of CTMABr decomposition were based on the kinetic model proposed by Flynn-Wall using thermal analysis of data performed with heating rates 5, 10, and 20 °C min−1.





Similar content being viewed by others
References
Meynen V, Cool P, Vasant EF. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009;125:170–223.
Klimova-Berestneva T, Martínez-Rosales JM, Ramírez-Solís J. Effect of surfactant removal method on the texture of Ti(R)-MCM-41. 2002;1:105–10.
Popova M, Szegedi Á, Németh P, Kostova N, Tsoncheva T. Peneiras moleculares titanium modified MCM-41 as a catalyst for toluene oxidation. Catal Commun. 2008;10:304–8.
Eimer GA, Chanquia CM, Sapag K, Herrero ER. The role of different parameters of synthesis in the final structure of Ti-containing mesoporous materials. Microporous Mesoporous Mater. 2008;116:670–76.
Wang JA, Chen LF, Norena, Navarrete LEJ, Llanos ME, Contreras JL, Novaro O. Mesoporous structure, surface acidity and catalytic properties of Pt/Zr–MCM-41 catalysts promoted with 12-tungstophosphoric acid. Microporous Mesoporous Mater. 2008;112:61–76.
Igarashi N, Hashimoto K, Tatsumi T. Catalytical studies on trimethylsilylated Ti-MCM-41 and Ti-MCM-48 materials. Microporous Mesoporous Mater. 2007;104:269–80.
Galacho C, Carrott MMLR, Carrott PJM. Structural and catalytic properties of Ti-MCM-41 synthesised at room temperature up to high Ti content. Micropor Mesopor Mater. 2007;100:312–21.
Gomes EL. Síntese de Peneiras Moleculares Contendo Nióbio ou Titânio e Aplicação em Epoxidação Catalítica. Universidade Federal de São Carlos Centro de Ciências Exatas e de Tecnologia. Programa de Pós-Graduação em Engenharia Química. São Carlos, Brasil 13 de Janeiro de 2005.
Braga RM, Barros JMF, Melo DMA, Melo MAF, Aquino FM, Freitas JCO, Santiago RC. Kinetic study of template removal of MCM-41 derived from rice husk ash. J Therm Anal Calorim. 2013;111:1013–8.
Macedo CP, Negrão CAB, Macedo LGM, Zamian JR, Rocha Filho GN, Costa CEF. Kinetic study of template removal of Al-MCM-41 synthesized at room temperature. J Therm Anal Calorim. 2013. doi:10.1007/s10973-013-3267-0.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetry data. J Polym Sci Part B: Polym Lett. 1966;4(5):323–8.
Souza MJB, Araújo AS, Pedrosa AMG, Lima SH, Fernades VH Jr. Kinetic parameters of the AlMCM-41 nanostructured materials. Thermochim Acta. 2006;443:183–8.
Aquino FM, Melo DMA, Santiago RC, Melo MAF, Martinelli AE, Freitas JCO, Araújo LCB. Thermal decomposition kinetics of PrMO3 (M = Ni or Co) ceramic materials via thermogravimetry. J Therm Anal Calorim. 2011;104:701–5.
Tuel A. Modification of mesoporous silicas by incorporation of heteroelements in the framework. Microporous Mesoporous Mater. 1999;27:151–69.
Brunauerm S, Emmet PH, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309–15.
Eimer GA, Casuscelli SG, Chanquia CA, Elías V, Crivello ME, Herrero ER. The influence of Ti-loading on the acid behavior and on the catalytic efficiency of mesoporous Ti-MCM-41 molecular sieves. Catal Today. 2008;133:639–46.
Souza MJB, Silva AOS, Aquino JMFB, Fernandes VJ, Araújo AS. Kinetic study of template removal of MCM-41 nanostructured material. J Therm Anal Calorim. 2004;75:693–8.
Barros JMF. Síntese e caracterização do Material nonoestruturado MCM-41 Contendo Terras Raras. Tese (Doutorado em Química). Natal: Universidade Federal do Rio Grande do Norte (UFRN); 2005. p. 30.
Araújo RS, Azevedo DCS, Rodríguez-Castellón E, Jiménez-López A, Cavalcante CL. Al and Ti-containing mesoporous molecular sieves: synthesis, characterization and redox activity in the anthracene oxidation. J Mol Catal A: Chem. 2008;281(1–2):154–63.
Blasco T, Camblor MA, Corma A, Pérez-Pariente J. The state of Ti in titanoaluminosilicates isomorphous with zeolite beta. J Am Chem Soc. 1993;115:11806–13.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1962;5:285–92.
Galacho PCGP. Materiais com estrutura mesoporosa ordenada contendo titânio: Preparação, Caracterização, Estudos de Adsorção e Propriedades Catalíticas, 2006. Évora: Universidade de Évora, Departamento de Química; 2005.
Acknowledgements
The authors would like to thank the Graduate Program in Materials Engineering (PPgCEM), CAPES, CNPq and LCR and Labtam Laboratories at UFRN and CEER-UFPB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fontes, M.S.B., Melo, D.M.A., Barros, J.M.F. et al. Kinetic study of CTMA+ removal from the pores of the Ti-MCM-41 molecular sieve. J Therm Anal Calorim 119, 197–204 (2015). https://doi.org/10.1007/s10973-014-4099-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4099-2