Abstract
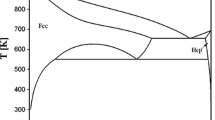
The results of calorimetric study of binary Al–Zn system done according to the Oelsen thermodynamic method are presented in this paper. Main thermodynamic properties, including activities, activity coefficients, partial/integral molar Gibbs excess, and mixing energies were determined at 1,000 K. Positive deviation from Raoult law was noticed, with minimal values of ΔG M about −3 kJ mol−1 and maximal values of ΔG E about +2 kJ mol−1. The energetics of mixing in liquid Al–Zn alloys has been analyzed through the study of concentration fluctuation in the long-wavelength limit, and weak affinity between Al and Zn atoms in the system was observed. Differential thermal analysis and light optic microscopy were applied in the frame of characterization of investigated binary alloys and phase diagram examination, and the results obtained were in accordance with available literature data.







Similar content being viewed by others
References
Živković D, Balanović L, Manasijević D, Mitovski A, Živković Ž, Kostić N. Calorimetric study of Al-Ga system using Oelsen method. Thermochim Acta. 2012;544:6–9. doi:10.1016/j.tca.2012.05.033.
Balanović L, Živković D, Manasijević D, Minić D, Marjanović B. Calorimetric study and thermal analysis of Al-Sn system. J Therm Anal Calorim. 2013;111(2):1431–5. doi:10.1007/s10973-012-2499-8.
Predel B, Arpshofen I, Pool MJ. Calorimetric methods in metallurgy. Thermochim Acta. 1978;22(2):211–36.
Balanović L, Živković D, Mitovski A, Manasijević D, Živković Ž. Calorimetric investigations and thermodynamic calculation of Zn-Al-Ga system. J Therm Anal Calorim. 2011;103(3):1055–61. doi:10.1007/s10973-010-1070-8.
Balanović L, Živković D, Manasijević D, Minić D, Živković Ž. Calorimetric investigation of Sn-Zn system. Metal Int. 2013;18(3):12–5.
Mazilkin AA, Straumal BB, Borodachenkova MV, Valiev RZ, Kogtenkova OA, Baretzky B. Gradual softening of Al-Zn alloys during high-pressure torsion. Mater Lett. 2012;84:63–5.
Chinh NQ, Csanádi T, Gyori T, Valiev RZ, Straumal BB, Kawasaki M, et al. Strain rate sensitivity studies in an ultrafine-grained Al-30 wt% Zn alloy using micro- and nanoindentation. Mater Sci Eng A. 2012;543:117–20. doi:10.1016/j.msea.2012.02.056.
Ares AE, Gassa LM, Schvezov CE, Rosenberger MR. Corrosion and wear resistance of hypoeutectic Zn-Al alloys as a function of structural features. Mater Chem Phys. 2012;136(2–3):394–414.
Rettenmayr M, Lambracht P, Kempf B, Tschudin C. Zn-Al based alloys as Pb-free solders for die attach. J Electron Mater. 2002;31(4):278–85.
Prasad LC, Mikula A. Thermodynamics of liquid Al-Sn-Zn alloys and concerned binaries in the light of soldering characteristics. Phys B. 2006;373(1):64–71. doi:10.1016/j.physb.2005.11.073.
Knott S, Mikula A. Thermodynamic properties of liquid Al-Sn-Zn alloys: a possible new lead-free solder material. Mater Trans. 2002;43(8):1868–72.
Hultgren R, Desai PD, Hawkins DT, Gleiser M, Kelley KK. Selected values of the thermodynamic properties of binary alloys. Metals Park, Ohio: American Society for Metals; 1973.
Predel B. Al-Zn (Aluminum-Zinc). In: Madelung O, editor. Phase equilibria, crystallographic and thermodynamic data of binary alloys: Ac-Au…Au-Zr. Landolt-Börnstein–Group IV Physical Chemistry. 1991. p. 1–6.
Murray JL. The Al-Zn (Aluminum-Zinc) system. Bull Alloy Phase Diagr. 1983;4(1):55–73. doi:10.1007/bf02880321.
Wittig FE, Keil G. Heats of mixing of binary liquid aluminum-B-metal alloys (Zinc, Cadmium, Indium, Thallium, Tin, Lead and Bismuth). Z Metallkde. 1963;54:576–90.
Hilliard JE, Auerbach BL, Cohen M. Thermodynamic properties of solid aluminum-zinc alloys. Acta Metall. 1954;2:621–31.
Corsepius H, Münster A. On the thermodynamic properties of solid aluminum-zinc alloys. Z Phys Chem. 1959;22:1–19.
Miller RE, Straalsund JL, Masson DB. Effect of electron concentration on the thermodynamic properties of two alloy phases in the Al-Zn-Ag system. Met Trans. 1972;3(2):545–50.
Piacente V, Di Paolo V, D’Ascenzo G. The activity of zinc in solid Al-Zn alloys. Thermochim Acta. 1976;16(1):63–8.
Takahashi T, Asano N. Thermodynamic studies of solid aluminum-zinc alloys. Niihama Kogyo Koto Senmo Gakko Kiyo, Rikogakn Hen. 1982;18:78.
Bolsaitis P, Sullivan PM. Activity of zinc in liquid Zn-Al alloys from isopiestic measurements. Met Soc AIME Trans. 1969;245(7):1435–8.
Yazawa A, Lee YK. Thermodynamic studies of the liquid aluminum alloys systems. Trans Jpn Inst Met. 1970;11:411–8.
Sebkova J, Beranek M. Application of the EMF method for measurement of aluminum activities in liquid aluminum alloys. Phys Sci Chem Technol Praze, Anorg Chem Technol B. 1974;18:217–25.
Janghorban A, Antoni-Zdziobek A, Lomello-Tafin M, Antion C, Mazingue T, Pisch A. Phase equilibria in the aluminium-rich side of the Al–Zr system. J Therm Anal Calorim. 2013;114(3):1015–20. doi:10.1007/s10973-013-3113-4.
Nayak AK. Thermodynamic analysis of Al-Zn alloys from calorimetric measurements. NML Techn J. 1981;23(1–2):3–10.
an Mey S, Effenberg G. A Thermodynamic evaluation of the Aluminium-Zinc system. Zeitschrift fuer Metallkunde/Mater Res Adv Tech. 1986;77(7):449–53.
an Mey S. Reevaluation of the Al-Zn system. Zeitschrift fuer Metallkunde/Mater Res Adv Tech. 1993;84(7):451–5.
Massalski TB, Murray JL, Bennett LH, Baker H. Binary alloy phase diagrams vol v. 2. metals park. Ohio: American Society for Metals; 1986.
Chen SL, Chang YA. A thermodynamic analysis of the Al-Zn system and phase diagram calculation. CALPHAD. 1993;17(2):113–24.
Aragon E, Jardet K, Satre P, Sebaoun A. The Al-Zn-Ga phase diagram: Part I. J Therm Anal Calorim. 1998;53(3):769–84.
Mathon M, Jardet K, Aragon E, Satre P, Sebaoun A. Al-Ga-Zn system: reassessments of the three binary systems and discussion on possible estimations and on optimisation of the ternary system. Comput Coupling Phase Diagr Thermochem. 2000;24(3):253–84. doi:10.1016/s0364-5916(01)00004-9.
Klančnik G, Medved J. Ternary invariant point at 374 °C in the three phase region AlSb-Al-Zn inside the Al-Sb-Zn ternary system. J Min Metall Sect B. 2011;47(2):179–92. doi:10.2298/jmmb110427013k.
Klančnik G, Medved J. Thermodynamic investigation of the Al-Sb-Zn system. Materiali Tehnologije. 2011;45(4):317–23.
Predel B. Al-Zn (Aluminum–Zinc). In: Predel B, editor. Ac-Ag… Au-Zr. Landolt-Börnstein–Group IV Physical Chemistry. Berlin: Springer; 2006. p. 1–2.
Drápala J, Kroupa A, Smetana B, Burkovič R, Lasek S, Musiol J. Study of Zn-Sn-Al alloys for high-temperature solders. METAL. 2009;19:21–5 (Hradec nad Moravicí2008).
Smetana B, Zlá S, Kroupa A, Žaludová M, Drápala J, Burkovič R, et al. Phase transition temperatures of Sn-Zn-Al system and their comparison with calculated phase diagrams. J Therm Anal Calorim. 2012;110(1):369–78. doi:10.1007/s10973-012-2318-2.
Cao Z, Xin J, Chen C, Liu S, Hu B, Tang C, et al. Thermodynamic modeling of the Bi-M (M = Ti, Cr, V) systems. J Min Metall Sect B. 2013;49(3):307–13. doi:10.2298/JMMB130127033C.
Oelsen W, Bieret F, Schwabe G. Zur thermodynamischen Analyse VI—Kalorimetrie und Thermodynamic der Wismut-Kadmium-Legierungen. Arch Eisenhuttenwess. 1956;27:607–20.
Oelsen W, Schurmann E, Weigt HJ, Oelsen O. Zur thermodynamischen Analyse IV—Vermischungsentropie und Bildungsaffinitat der Blei-Kadmium-Schmelzen aus kalorimetrischen Messungen. Arch Eisenhuttenwess. 1956;27:487–511.
Oelsen W, Zuhlke P. Zur thermodynamischen analyse VIII. Arch Eisenhuttenwess. 1956;27:743–54.
Živković D, Katayama I, Gomidželović L, Manasijević D, Novaković R. Comparative thermodynamic study and phase equilibria of the Bi-Ga-Sn ternary system. Int J Mater Res. 2007;98(10):1025–30. doi:10.3139/146.101561.
Živković D, Mitovski A, Balanović L, Manasijević D, Živković Ž. Thermodynamic analysis of liquid In-Sn alloys using Oelsen calorimetry. J Therm Anal Calorim. 2010;102(3):827–30. doi:10.1007/s10973-010-0785-x.
Singh RN. Short range order and concentration fluctuations in binary molten alloys. Special issue–liquid metals and alloys. Can J Phys. 1987;65:309–25.
Bhatia AB, Singh RN. Short range order and concentration fluctuations in regular and compound forming molten alloys. Phys Chem Liq. 1982;11(4):285–313.
Acknowledgements
The authors acknowledge the support of Ministry of Education, Science and Technological Development, Republic of Serbia under the project OI172037.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balanović, L., Živković, D., Manasijević, D. et al. Calorimetric investigation of Al–Zn alloys using Oelsen method. J Therm Anal Calorim 118, 1287–1292 (2014). https://doi.org/10.1007/s10973-014-3990-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3990-1