Abstract
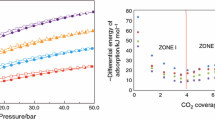
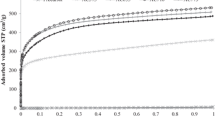
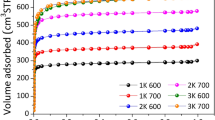
Given the great interest in the CO2 removal and decreasing their impact on the environment, in this work, a calorimetric study of CO2 adsorption on different activated carbons was performed. For this purpose, we used two methodologies for the determination heat of CO2 adsorption: determination of CO2 isotherms at different temperatures and adsorption calorimetry. The heats determined by these two techniques were compared. In this regard, carbonaceous materials of granular and monolithic types were prepared, characterized, and functionalized for carbon dioxide adsorption. As precursor material, African palm stones that were activated with H3PO4 and CaCl2 at different concentrations was used. The obtained materials were functionalized in gas phase with NH3 and liquid phase with NH4OH, with the intention to incorporate the surface basic groups (amines or nitrogen groups) and subsequently were studied for CO2 adsorption at 273 K and atmospheric pressure. For characterization of these materials, the following techniques are used: N2 adsorption at 77 K and immersion calorimetry in different solvents. The experimental results show the obtaining of micropores and mesoporous (moderately) materials, with surface area between 430 and 1,425 m2 g−1 and pore volumes between 0.17 and 0.53 cm3 g−1. It was determined that there is a difference between the heats of CO2 adsorption obtained by the techniques employed. This deviation between the values corresponds to the methodological difference between the two experiments. In this work, we obtained a maximum adsorption capacity of CO2, which is greater than 334 mg CO2 g−1 at 273 K and 1 bar in carbon materials with moderate surface area and pores volume.







Similar content being viewed by others
References
Bandyopadhyay A. Amine versus ammonia absorption of CO2 as a measure of reducing GHG emission: a critical analysis. Clean Technol Environ Policy. 2001;13(2):269–94.
Yang H, Xu Z, Fan M, Gupta R, Slimane RB, Bland AE, Wright I. Progress in carbon dioxide separation and capture: a review. J. Environ. Sci. 2008;20(1):14–27.
García S, Gil MV, Martín CF, Pis JJ, Rubiera F, Pevida C. Breakthrough adsorption study of a commercial activated carbon for pre-combustion CO2 capture. Chem Eng J. 2011;171(2):549–56.
Thote JA, Iyer KS, Ravikrishna C, Labhsetwar N, Biniwale RB, Rayalu SS. In situ nitrogen enriched carbon for carbon dioxide capture. Carbon. 2010;48(2):396–402.
Radosław P, Wesołowski PA, Gauden AP, Terzyk SF. The applicability of the numerical algorithm for the evaluation of isosteric heat of adsorption. Carbon. 2004;42(1):53–8.
Vargas DP, Giraldo L, Moreno-Piraján JC. Study of CO2 adsorption in functionalized carbon. Adsorption. 2013;19(2):323–9.
Nakagawa Y, Molina-Sabio M, Rodríguez-Reinoso F. Modification of the porous structure along the preparation of activated carbon monoliths with H3PO4 and ZnCl2. Microporous Mesoporous Mater. 2007;103(1):29–34.
Juárez-Galán JM, Silvestre-Albero A, Silvestre-Albero J, Rodríguez-Reinoso F. Synthesis of activated carbon with highly developed mesoporosity. Microporous Mesoporous Mater. 2009;117(1):519–21.
Vargas DP, Giraldo L, Moreno-Piraján JC. CO2 adsorption on granular and monolith carbonaceous materials. J Anal Appl Pyrolysis. 2012;96:146–52.
Pirngruber GD, Cassiano-Gaspar S, Louret S, Chaumonnot A, Delfort B. Amines immobilized on a solid support for postcombustion CO2 capture–A preliminary analysis of the performance in a VSA or TSA process based on the adsorption isotherms and kinetic data. Energy Proced. 2009;1(1):1335–42.
Plaza MG, García S, Rubiera F, Pis JJ, Pevida C. Evaluation of ammonia modified and conventionally activated biomass based carbons as CO2 adsorbents in postcombustion conditions. Sep Purif Technol. 2011;80(1):96–104.
Plaza MG, Pevida C, Arias B, Fermoso J, Rubiera F, Pis JJ. CO2 capture by adsorption with nitrogen enriched carbons. Fuel. 2007;86(14):2204–12.
Plaza MG, Pevida C, Arias B, Fermoso J, Rubiera F, Pis JJ. A comparison of two methods for producing CO2 capture adsorbents. Energy Proced. 2009;1(1):1013–107.
Przepiòrski J, Skrodzewicz M, Morawski AW. High temperature ammonia treatment of activated carbon for enhancement of CO2 adsorption. Appl Surf Sci. 2004;225(1):235–42.
Marsh H, Rodríguez-Reinoso F. Characterization of activated carbon. Activated carbon. Amsterdam: Elsevier Science Ltd.; 2005. p. 157–64 ISBN: 0080444636.
Silvestre-Albero J, Gómez C, Sepúlveda-Escribano A, Rodríguez-Reinoso F. Characterization of microporous solids by Immersion calorimetry. Coll Surf A. 2001;187–188:151–65.
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem. 1985;57(4):603–19.
IUPAC. Physisorption of Gases, with special reference to the evaluation of surface area and pore size distribution. Chem Int. 2011;33:23.
Garrido J, Linares-Solano A, Martín-Martínez JM, Molina-Sabio M, Rodríguez-Reinoso F, Torregrosa R. Use of nitrogen vs. carbon dioxide in the characterization of activated carbons. Langmuir. 1987;3(1):76–81.
Pevida C, Plaza MG, Arias B, Fermoso J, Rubiera F, Pis JJ. Surface modification of activated carbons for CO2 capture. Appl Surf Sci. 2008;254(22):7165–72.
Mangun CL, Benak KR, Economy J, Foster KL. Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon. 2001;39(12):1809–20.
Szekely J, Evans JW, Sohn HY. Gas-solid reactions. New York: Academic Press; 1976.
Águeda VI, Crittenden BD, Delgado JA, Tennison SR. Effect of channel geometry, degree of activation, relative humidity and temperature on the performance of binderless activated carbon monoliths in the removal of dichloromethane from air. Sep Purif Technol. 2011;78(2):154–63.
Zhao Z, Cui X, Ma J, Li R. Adsorption of carbon dioxide on alkali-modified zeolite 13X adsorbents. Int J Greenh Gas Control. 2007;1(3):355–9.
Sabouni R, Kazemian H, Rohani S. Carbon dioxide adsorption in microwave-synthesized metal organic framework CPM-5: equilibrium and kinetics study. Microporous Mesoporous Mater. 2013;175:85–91.
Ana H, Fenga B, Sub S. Effect of monolithic structure on CO2 adsorption performance of activated carbon fiber–phenolic resin composite: a simulation study. Fuel. 2013;103:80–6.
Hao W, Björkman E, Lilliestråle M, Hedin N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl Energy. 2013;112:526–32.
Sevilla M, Fuertes AB. CO2 adsorption by activated templated carbons. J Coll Interface Sci. 2012;366(1):147–54.
Yang ST, Kim JY, Kim J, Ahn WS. CO2 capture over amine-functionalized MCM-22, MCM-36 and ITQ-2. Fuel. 2012;97:435–42.
Wahby A, Silvestre-Albero J, Sepúlveda-Escribano A, Rodríguez-Reinoso F. CO2 adsorption on carbon molecular sieves. Microporous Mesoporous Mater. 2012;164:280–7.
Wahby A, Ramos-Fernández JM, Martínez-Escandell M, Sepúlveda-Escribano A, Silvestre-Albero J, Rodríguez-Reinoso F. High-surface-area carbon molecular sieves for selective CO2 adsorption. ChemSusChem. 2010;3(8):974–81.
Cho HY, Yang DA, Kim J, Jeong SY, Ahn WS. CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating. Catal Today. 2012;185(1):35–40.
Plaza MG, Rubiera F, Pis JJ, Pevida C. Ammoxidation of carbon materials for CO2 capture. Appl Surf Sci. 2010;256(22):6843–9.
Acknowledgements
The authors thank the Framework Agreement between Universidad de los Andes and Universidad Nacional de Colombia, as well as the Agreement Statement (Acta de Acuerdo) between the Departments of Chemistry of both Universities. The authors are especially grateful to the Vice-Rectory of Research and the Faculty of Sciences at the University of Andes for funding for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
The present article is based on the lecture presented at II Workshop on Adsorption, Catalysis and Porous Materials in Bogotá—Colombia on 27–31 May, 2013.
Rights and permissions
About this article
Cite this article
Vargas, D.P., Giraldo, L. & Moreno-Piraján, J.C. Calorimetric study of the CO2 adsorption on carbon materials. J Therm Anal Calorim 117, 1299–1309 (2014). https://doi.org/10.1007/s10973-014-3909-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3909-x