Abstract
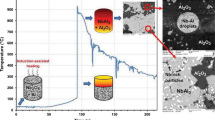
Thermite reactions between aluminum and metal oxides could lead to the formation of intermetallic matrix composites used in high-temperature industrial applications. Thermite reaction in Al–TiO2 system needs a considerable amount of energy to take place by mechanochemical or by the combustion synthesis (CS) method due to the low amount of reaction enthalpy in Al–TiO2 system. In this study, Fe2O3 was chosen as a accelerator for this system, to generate a high amount of heat which could be released between Fe2O3 and Al, leading to a more convenient reaction between Al and TiO2 in the CS process. The results of XRD, SEM, and DSC analyses indicated that both the mechanical activation of Al–TiO2 system in a high-energy ball mill and the Fe2O3 addition led to considerable effects of reduction in the reaction temperature and increase in the reaction intensity in Al–TiO2 nanothermite system. Finally, it was shown that Fe3Al intermetallic compounds as well as γ-AlTi and alumina phases in the final products were formed after the CS of the milled powders.








Similar content being viewed by others
References
Kumar KS, Bao G. Intermetallic matrix composites: an overview. Compos Sci Technol. 1994;52:127–50.
Ward-Close CM, Minor R, Doorbar PJ. Intermetallic-matrix composites: a review. Intermetallics. 1996;4:217–29.
Koch CC. Intermetallic matrix composites prepared by mechanical alloying—a review. Mater Sci Eng A. 1998;244:39–48.
Johnson DR. Intermetallic-based composites. Curr Opin Solid State Mater Sci. 1999;4:249–53.
Gong H, Yin Y, Wang X, Liu Y. Fabrication and microstructure of in situ toughened Al2O3/Fe3Al. Mater Res Bull. 2004;39:513–21.
Schicker S, Garcia DE, Bruhn J, Janssen R, Claussen N. Reaction synthesized Al2O3-based intermetallic composites. Acta Mater. 1998;46(7):2485–92.
Mousavian RT, Sharafi S, Shariat MH. Preparation of nano-structural Al2O3–TiB2 in situ composite using mechanically activated combustion synthesis followed by intensive milling. Iran J Mater Sci Eng. 2011;8(2):1–9.
Mousavian RT, Sharafi S, Roshan MR, Shariat MH. Effect of mechanical activation of reagents’ mixture on the high-temperature synthesis of Al2O3–TiB2 composite powder. J Therm Anal Calorim. 2011;104(3):1063–70.
Mousavian RT, Sharafi S, Shariat MH. Microwave-assisted combustion synthesis in a mechanically activated Al–TiO2–H3BO3 system. Int. J Refract Met Hard Mater. 2011;29:281–8.
Deris L, Sharafi S, Akbari GH. Effect of milling speed on mechanical activation of Al/ZrO2/H3BO3 system to prepare Al2O3–ZrB2 composite powder. J Therm Anal Calorim. 2014;115:401–7.
Hasani S, Panjepour M, Shamanian M. Effect of atmosphere and heating rate on mechanism of MoSi2 formation during self-propagating high-temperature synthesis. J Therm Anal Calorim. 2012;107:1073–81.
Comet M, Siegert B, Pichot V, Spitzer D. Reactive characterization of nanothermites, correlation structure/reactivity. J Therm Anal Calorim. 2013;111:431–6.
Stojanovic BD, Marinkovic ZV, Brankovic GO, Fidanevska E. Evaluation of kinetic data for crystallization of TiO2 prepared by hydrolysis method. J Therm Anal Calorim. 2000;60:595–604.
Sorescu M, Xu T. The effect of ball-milling on the thermal behavior of anatase-doped hematite ceramic system. J Therm Anal Calorim. 2011;103:479–84.
Wiezorek-Ciurowa K, Gamrat K, Sawlowicz Z. Characteristics of CuAl2–Cu9Al4/Al2O3 nanocomposites synthesized by mechanical treatment. J Therm Anal Calorim. 2005;80:619–23.
Wiezorek-Ciurowa K, Gamrat K. NiAl/Ni3Al–Al2O3 composite formation by reactive ball milling. J Therm Anal Calorim. 2005;82:719–24.
Patoya ML, Granier JJ. The effect of slow heating rates on the reaction mechanisms of nano and micron composite thermite reactions. J Therm Anal Calorim. 2006;85(1):37–43.
Shen YF, Zou ZG, Xiao ZG, Liu K, Long F, Wu Y. Properties and electronic structures of titanium aluminides–alumina composites from in situ SHS process. Mater Sci Eng A. 2011;528:2100–5.
Hsu HW, Chao CG. Effect of heat treatments on in situ Al2O3/TiAl3 composites produced from squeeze casting of TiO2/A356 composites. Metall Mater Trans B. 2002;33:31–40.
Ying DY, Zhang DL, Newby M. Solid-state reactions during heating mechanically milled Al/TiO2 composite powders. Metall Mater Trans A. 2004;35:2115–25.
Feng CF, Froyen L. Formation of Al3Ti and Al2O3 from an Al–TiO2 system for preparing in situ aluminium matrix composites. Compos A. 2000;31:385–90.
Gheorghe I, Rack HJ. Reactive infiltration of 25 vol Pct TiO2/Al composites. Metall Mater Trans A. 2002;33:2155–62.
Welham NJ. Mechanical activation of the solid-state reaction between Al and TiO2. Mater Sci Eng A. 1998;255:81–9.
Alamolhoda S, Heshmati-Manesh S, Ataie A. Role of intensive milling in mechano-thermal processing of TiAl/Al2O3 nano-composite. Adv Powder Technol. 2012;23(3):343–8.
Kamali AR, Babaei-Nejad I, Aboutalebi MR, Razavizadeh H. Effect of ball milling on reaction between TiO2 and Al. Russ J Non-Ferr Met. 2009;50(3):246–9.
Liu Z, Raynova S, Zhang GDB. Study on the self sustained reactions in an Al–TiO2 composite powder produced by high-energy mechanical milling. Mater Sci Eng A. 2007;449–51:1107–10.
Bodaghi M, Zolfonoon H, Tahriri M, Karimi M. Synthesis and characterization of nanocrystalline α-Al2O3 using Al and Fe2O3 (hematite) through mechanical alloying. Solid State Sci. 2009;11:496–500.
Yadav TP, Yadav RM, Singh DP. Mechanical milling: a top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci Nanotechnol. 2012;2(3):22–48.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousavian, R.T., Azizi, N., Jiang, Z. et al. Effect of Fe2O3 as an accelerator on the reaction mechanism of Al–TiO2 nanothermite system. J Therm Anal Calorim 117, 711–719 (2014). https://doi.org/10.1007/s10973-014-3820-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3820-5