Abstract
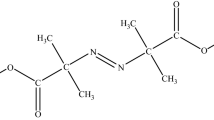
1,1-bis(tert-Butylperoxy)-3,3,5-trimethylcyclohexane (TMCH) is commonly used as a crosslinking agent or an initiator of the heat-curing agent for polybutadiene rubber. Metal ions that remain in the pipelines or containers of manufacturing processes may affect the thermal stability of the organic peroxides. Moreover, pipelines or metal containers may contain some metal ions because of inner corrosive chemicals or surface deterioration, which may induce a chemical reaction, while TMCH is mixed with them. To avoid these unexpected chemical reactions, we focused on the thermal hazard analysis of TMCH mixed with metal ions, such as nickel(II) bromide or copper(II) bromide. The experiments can determine thermokinetic parameters, including exothermic onset temperature (T 0), maximum temperature (T max), and heat of decomposition (ΔH d), under non-isothermal conditions by differential scanning calorimetry. Non-isothermal experimental results combined with isoconversional kinetic analysis can acquire further safety parameters, such as apparent activation energy (E a) and time to maximum heating rate. The results of this study could be used as a proactive case for the storage of TMCH mixed with metal ions.















Similar content being viewed by others
Abbreviations
- A :
-
Frequency factor (s−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- k :
-
Heat-transfer coefficient (W m−1 K−1)
- k i :
-
Rate constant (mol L−1s−1) (i = 1, 2)
- t :
-
Time (s)
- T :
-
Absolute temperature (K)
- T 0 :
-
Exothermic onset temperature (°C)
- T αi :
-
Different temperature in various heating rates at isoconversional degree αi; i = 0.5, 1, 2, 4, 8
- TMRiso :
-
Time to maximum heating rate under isothermal conditions (min)
- R :
-
Gas constant (8.31415 J K−1 mol−1)
- β :
-
Heating rate (°C min−1)
- ∆H d :
-
Heat of decomposition (J g−1)
References
Tseng JM, Shu CM, Gupta JP, Lin YF. Evaluation and modeling runaway reaction of methyl ethyl ketone peroxide mixed with nitric acid. Ind Eng Chem Res. 2007;46:8738–45.
Lin YF, Lin CP, Chen LY, Su TS, Tseng JM. Effect of different concentrations of acetone for the decomposition reactions of peroxyketal peroxides. Thermochim Acta. 2012;527:27–32.
Tseng JM, Lin CP, Huang ST, Hsu J. Kinetic and safety parameters analysis for 1,1-di-(tert-butylperoxy)-3,3,5-trimethylcyclohexane in isothermal and non-isothermal conditions. J Hazard Mater. 2011;192:1427–36.
Duh YS, Wu XH, Kao CS. Hazard ratings for organic peroxides. Process Saf Prog. 2008;27(2):89–99.
Li AC, Tsai YT, Wu SH, Chiu CW, Shen SJ, Hsin R, Shu CM. Thermal runaway analysis for two organic peroxides with H2O and dry fire-extinguishing chemicals by DSC and VSP2. J Therm Anal Calorim. 2013;113:1611–8.
Wang TS, Liu SH, Qian XM, Shu CM. Isothermal hazards evaluation of benzoyl peroxide mixed with benzoic acid via TAM III Test. J Therm Anal Calorim. 2013;113:1625–31.
Weng SY, Liu SH, Tsai LC, Hsieh TF, Ma CM, Shu CM. Thermokinetics simulation for multi-walled carbon nanotubes with sodium alginate by advanced kinetics and technology solutions. J Therm Anal Calorim. 2013;113:1603–10.
Liu SH, Hou HY, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids and alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;556:226–32.
Tsai YT, You ML, Qian XM, Shu CM. Calorimetric techniques combined with various thermokinetic models to evaluate incompatible hazard of tert-butyl peroxy-2-ethyl hexanoate mixed with metal ions. Ind Eng Chem Res. 2013;52:8206–15.
Tseng JM, Chang YY, Su TS, Shu CM. Study of thermal decomposition of methyl ethyl ketone peroxide using DSC and simulation. J Hazard Mater. 2007;142:765–70.
Belichmeier JA, Cammenga HK, Schneider PB, Steer AG. A simple method for determining activation energies of organic reactions from DSC curves. Thermochim Acta. 1998;310:147–51.
Yang D, Koseki H, Hasegawa K. Predicting the self-accelerating decomposition temperature (SADT) of organic peroxides based on non-isothermal decomposition behavior. J Loss Prev Process Ind. 2003;16(5):411–6.
Chu YC, Tsai FC, Lin FR, Chiang TC, Shu CM. Evaluation unexpected energy released for three liquid organic peroxides. Energy Educ Sci Tech Pt A. 2013;30:977–82.
Jhu CY, Wang YW, Wen CY, Shu CM. Thermal runaway potential of LiCoO2 and Li(Ni1/3Co1/3Mn1/3)O2 batteries determined with adiabatic calorimetry methodology. Appl Energy. 2012;100:127–31.
Lu TY, Chen JL, Chi JH, Wu SH, Jian HL. Thermal explosion analysis of tert-butyl peroxide by calorimetric technology and mathematical model development. J Appl Fire Sci. 2012;21(2):85–97.
Zang N, Qian XM, Liao JY, Shu CM. Thermal stability of lauroyl peroxide by isoconversional kinetics evaluation and finite element analysis. J Taiwan Inst Chem Eng. 2014;45:461–7.
Chen CJ, Chi JH, Wu SH, Chen CT, Tsai HF. Fundamental thermal hazard investigation for tert-butyl peroxide reactor using DSC and TGA techniques. J Appl Fire Sci. 2012;21(1):53–63.
Lee MJ, Wen IJ, Wu SH, Shu CM. Fire and explosion prevention of two organic peroxides combined with five fire-extinguishing agents. J Appl Fire Sci. 2011;20(2):135–48.
Lee MH, Chen JR, Shiue GY, Lin YF, Shu CM. Simulation approach to benzoyl peroxide decomposition kinetics by thermal calorimetric technique. J Taiwan Inst Chem E. 2014;45:115–20.
American Society for Testing and Materials (ASTM). E698 Standard test method for Arrhenius kinetic constants for thermally unstable materials. West Conshohocken, PA, USA. 2001.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–7.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–24.
Ozawa T. Estimation of activation energy by isoconversion methods. Thermochim Acta. 1992;203:159–65.
Ozawa T. Thermal analysis—review and prospect. Thermochim Acta. 1999;355:35–42.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci B. 1966;4(5):323–8.
Roduit B, Xia L, Folly P, Berger B, Mathieu J, Sarbach A, Andres H, Ramin M, Vogelsanger B, Spitzer D, Moulard H, Dilhan D. The simulation of the thermal behavior of energetic materials based on DSC and HFC signals. J Therm Anal Calorim. 2008;93(1):143–52.
Acknowledgements
The authors wish to thank Ms. Vaefun Shi and Mr. Ao Chen for their assistance in solving the problems from simulations by differential isoconversional kinetic analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, WC., Chen, WT., Wang, YW. et al. Effects of mixing metal ions for the thermal runaway reaction of TMCH. J Therm Anal Calorim 118, 1003–1010 (2014). https://doi.org/10.1007/s10973-014-3813-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3813-4