Abstract
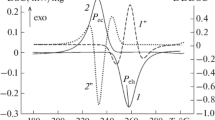
A three-step method to determine the eutectic composition of a binary or ternary mixture is introduced. The method consists in creating a temperature–composition diagram, validating the predicted eutectic composition via differential scanning calorimetry and subsequent T-History measurements. To test the three-step method, we use two novel eutectic phase change materials based on \(\mathrm{Zn}(\hbox {NO}_3)_2\cdot 6\mathrm{H}_{2}{\mathrm O}\) and \(\mathrm{NH}_4\mathrm{NO}_3\) respectively \(\mathrm{Mn}(\hbox {NO}_3)_2\cdot 6\mathrm{H}_{2}{\hbox {O}}\) and \(\mathrm{NH}_4\mathrm{NO}_3\) with equilibrium liquidus temperatures of 12.4 and 3.9 \(\,^{\circ }\mathrm {C}\) respectively with corresponding melting enthalpies of 135 J \(\mathrm{g}^{-1}\) (237 J \(\mathrm{cm}^{-3}\)) respectively 133 J \(\mathrm{g}^{-1}\) (225 J \(\mathrm{cm}^{-3}\)). We find eutectic compositions of 75/25 mass% for \(\mathrm{Zn}(\hbox {NO}_3)_2\cdot \mathrm{6H}_{2}{\mathrm{O}}\) and \(\mathrm{NH}_4\mathrm{NO}_3\) and 73/27 mass% for \(\mathrm{Mn}(\hbox {NO}_3)_2\cdot 6\mathrm{H}_{2}{\mathrm{O}}\) and \(\mathrm{NH}_4\mathrm{NO}_3\). Considering a temperature range of 15 K around the phase change, a maximum storage capacity of about 172 J \(\mathrm{g}^{-1}\) (302 J \(\mathrm{cm}^{-3}\)) respectively 162 J \(\mathrm{g}^{-1}\) (274 J \(\mathrm{cm}^{-3}\)) was determined for \(\mathrm{Zn}(\hbox {NO}_3)_2\cdot \mathrm{6H}_{2}{\mathrm{O}}\) and \(\mathrm{NH}_4\mathrm{NO}_3\) respectively \(\mathrm{Mn}(\hbox {NO}_3)_2\cdot \mathrm{6H}_{2}{\mathrm{O}}\) and \(\mathrm{NH}_4\mathrm{NO}_3\).








Similar content being viewed by others
References
Mehling H, Cabeza LF. Heat and cold storage with PCM. Berlin: Springer; 2008.
Jeon J, Lee J-H, Seo J, Jeong S-G, Kim S. Application of pcm thermal energy storage system to reduce building energy consumption. J Therm Anal Calorim. 2013;111:279–88.
Zhai XQ, Wang XL, Wang T, Wang RZ. A review on phase change cold storage in air conditioning system: materials and applications. Renew Sust Energy Rev. 2013;22:108–20.
Cabeza LF, Castell A, Barreneche C, de Gracia A, Fernández AI. Materials used as pcm in thermal energy storage in buildings: a review. Renew Sust Energy Rev. 2011;15:1675–95.
Farid MM, Khudhair AM, Siddique AKR, Said A-H. A review on phase energy storage: materials and applications. Eenergy Convers Manage. 2004;45(15):97–1615.
Nagano K, Mochida T, Takeda S, Domnski R, Rebow M. Thermal characteristics of manganese (ii) nitrate hexahydrate as a phase change material for cooling systems. Appl Therm Eng. 2003;23:229–41.
Lane GA. Solar heat storage: latent heat material, vol. II. Boca Raton: CRC Press, Inc.; 1986.
Benrath A, Hartung P, Wilden M. Über die anwendung der auftau-schmelzmethode auf anorganische binäre systeme. J Prakt Chem. 1935;143:298–304.
Marcus Y, Minevich A, Ben-Dor L. Solid–liquid phase diagram of binary salt hydrate mixtures involving magnesium nitrate and acetate, magnesium and aluminum nitrate, ammonium alum and sulfate, and ammonium alum and aluminum sulfate. Thermochim Acta. 2004;412:163–70.
Marcus Y, Minevich A, Ben-Dor L. Solid–liquid phase equilibria of binary salt hydrate mixtures involving ammonium alum. J Therm Anal Calorim. 2005;81:51–5.
Benessam S, Khimeche K, Djellouli F, Benziane M, Dahmani A. Phase diagram of ibuprofen with fatty acids. J Therm Anal Calorim. 2013;112:317–20.
Rycerz L. Practical remarks concerning phase diagram determination on the basis of differential scanning calorimetry measurements. J Therm Anal Calorim. 2013;113:231–8.
Günther E, Hiebler S, Mehling H, Redlich R. Enthalpy of phase change materials as a function of temperature: required accuracy and suitable measurement methods. Int J Thermophys. 2009;30:1257–69.
Kousksou T, Jamil A, Zeraouli Y, Dumas J-P. Equilibrium system liquidus temperatures of binary mixtures from differential scanning calorimetry. Chem Eng Sci. 2007;62:6516–23.
Ewing WW, McGovern JJ, Mathews GE. The temperature–composition relations of the binary system zinc nitrate–water. J Am Chem Soc. 1933;55:4827–30.
Ibnlfassi A, Kaddami D, El Kacemi K. Systme ternaire: \(\text{H}_{2}\text{O}-\text{Zn}(\text{NO}_3)_2-\text{NH}_4\text{NO}_3\) i. les isothermes \(-\)25 et \(-\)20\(\rm ^\circ \)C. J Therm Anal Calorim. 2003;74:341–7.
Purdon FF, Slater VW. Aqueous solution and the phase diagram. London: Edward Arnold & CO; 1946.
Dellien I. A dsc study of the phase transformations of ammonium nitrate. Thermochim Acta. 1982;55:181–91.
24 LEA, SHC Task 42/ECES Annex. Compact thermal energy storage. http://task42.iea-shc.org/
Lázaro A, Günther E, Mehling H, Hiebler S, Marín JM, Zalba B. Verification of a t-history installation to measure enthalpy versus temperature curves of phase change materials. Meas Sci Technol. 2006;17:2168–74.
Rathgeber C, Schmit H, Hennemann P, Hiebler S. Calibration of a t-history calorimeter to measure enthalpy curves of phase change materials in the temperature range from 40 to 200 ºC. Meas Sci Technol. 2014;25:035011.
Abhat A. Low temperature latent heat storage: I: heat storage materials; II: heat transfer considerations. Ispra: Ispra Courses; 1981.
Acknowledgements
This work is part of the project EnFoVerM and was supported by the German Federal Ministry of Economics and Technology under the project code 0327851D. The responsibility for the content of this publication is with the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmit, H., Rathgeber, C., Hennemann, P. et al. Three–step method to determine the eutectic composition of binary and ternary mixtures. J Therm Anal Calorim 117, 595–602 (2014). https://doi.org/10.1007/s10973-014-3783-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3783-6