Abstract
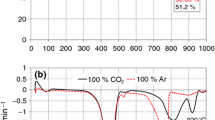
A possible technology that can contribute reduction of carbon dioxide emission is oxy-fuel combustion of fossil fuels enabling to increase CO2 concentration in the exhaust gas by carrying out the combustion process with oxygen and replacing air nitrogen with recycling combustion products to obtain a capture-ready CO2 stream. The laboratory studies and pilot-scale experiments discussed during the last years have indicated that oxy-fuel combustion is a favorable option in retrofitting conventional coal firing. Estonian oil shale (OS) with its specific properties has never been studied as a fuel in oxy-fuel combustion, so, the aim of the present research was to compare thermo-oxidation of OS and some coal samples under air and oxy-fuel combustion conditions by means of thermal analysis methods. Experiments were carried out in Ar/O2 and CO2/O2 atmospheres with two oil shale and two coal samples under dynamic heating conditions. FTIR analysis was applied to characterize evolved gases and emission dynamics. Kinetic parameters of oxidation were calculated using a model-free kinetic analysis approach based on differential iso-conversional methods. Comparison of the oxidation characteristics of the samples was given in both atmospheres and it was shown that the oxidation process proceeds under oxy-fuel conditions by all studied fuels with lower activation energies, however, it can last longer as the same temperatures are compared.





Similar content being viewed by others
References
Climate Change 2007: The physical science basis. Summary for policymakers. IPCC Fourth Assessment Report of the intergovernmental panel on climate change. Approved at the 10th session of working group I of the IPCC, Paris, Feb 2007.
IPCC expert meeting on detection and attribution related to anthropogenic climate change. The world meteorological organization. Geneva, Switzerland, 14–16 Sep 2009.
Davison J, Thambimuthu K, Rubin ES, Keith DW, Gilboy CF, Wilson M, et al. Technologies for capture of carbon dioxide. Greenhouse gas control technologies 7. Oxford: Elsevier; 2005. p. 3–13.
Lyngfelt A, Leckner B, editors. Technologies for CO2 sequestration. Minisymposium on carbon dioxide capture and storage, School of Environmental Sciences, October 22, 1999. Gothenburg: Chalmers University of Technology; 1999.
Wall T, Liu Y, Spero C, Elliott L, Khare S, Rathnam R, et al. An overview on oxyfuel coal combustion–State of the art research and technology development. Chem Eng Res Des. 2009;87(8):1003–16.
Buhre BJP, Elliott LK, Sheng CD, Gupta RP, Wall TF. Oxy-fuel combustion technology for coal-fired power generation. Prog Energy Combust Sci. 2005;31(4):283–307.
Anheden M, Burchhardt U, Ecke H, Faber R, Jidinger O, Giering R, et al. Overview of operational experience and results from test activities in Vattenfall’s 30 MWth oxyfuel pilot plant in schwarze pumpe. Energy Procedia. 2011;4(0):941–50. doi:10.1016/j.egypro.2011.01.140.
Kluger F, Prodhomme B, Mönckert P, Levasseur A, Leandri J-F. CO2 capture system—confirmation of oxy-combustion promises through pilot operation. Energy Procedia. 2011;4(0):917–24.
Lupion M, Diego R, Loubeau L, Navarrete B. CIUDEN CCS project: status of the CO2 capture technology development plant in power generation. Energy Procedia. 2011;4(0):5639–46. doi:10.1016/j.egypro.2011.02.555.
Lupion M, Navarrete B, Otero P, Cortés VJ. Experimental programme in CIUDEN’s CO2 capture technology development plant for power generation. Chem Eng Res Des. 2011;89(9):1494–500.
Stadler H, Christ D, Habermehl M, Heil P, Kellermann A, Ohliger A, et al. Experimental investigation of NO x emissions in oxycoal combustion. Fuel. 2011;90(4):1604–11.
Krzywanski J, Czakiert T, Muskala W, Nowak W. Modelling of CO2, CO, SO2, O2 and NO x emissions from the oxy-fuel combustion in a circulating fluidized bed. Fuel Process Technol. 2011;92(3):590–6.
Liszka M, Ziebik A. Coal-fired oxy-fuel power unit–process and system analysis. Energy. 2010;35(2):943–51.
Seepana S, Jayanti S. Optimized enriched CO2 recycle oxy-fuel combustion for high ash coals. Fuel. 2012;102(2012):32–40. doi:10.1016/j.fuel.2009.04.029.
Toftegaard MB, Brix J, Jensen PA, Glarborg P, Jensen AD. Oxy-fuel combustion of solid fuels. Prog Energy Combust Sci. 2010;36(5):581–625. doi:10.1016/j.pecs.2010.02.001.
Varheyi G, Till F. Comparison of temperature-programmed char combustion in CO2–O2 and Ar–O2 mixtures at elevated pressure. Energy Fuels. 1999;13:539–40.
Heil P, Toporov D, Förster M, Kneer R. Experimental investigation on the effect of O2 and CO2 on burning rates during oxyfuel combustion of methane. Proc Combust Inst. 2011;33(2):3407–13.
Niu S, Lu C, Han K, Zhao J. Thermogravimetric analysis of combustion characteristics and kinetic parameters of pulverized coals in oxy-fuel atmosphere. J Therm Anal Cal. 2009;98(1):267–74. doi:10.1007/s10973-009-0133-1.
Gil M, Riaza J, Álvarez L, Pevida C, Pis J, Rubiera F. A study of oxy-coal combustion with steam addition and biomass blending by thermogravimetric analysis. J Therm Anal Cal. 2012;109(1):49–55. doi:10.1007/s10973-011-1342-y.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201(4914):68–9.
Dhaneswar SR, Pisupati SV. Oxy-fuel combustion: the effect of coal rank and the role of char-CO2 reaction. Fuel Process Technol. 2012;102:156–65. doi:10.1016/j.fuproc.2012.04.029.
Al-Makhadmeh L, Maier J, Al-Harahsheh M, Scheffknecht G. Oxy-fuel technology: an experimental investigations into oil shale combustion under oxy-fuel conditions. Fuel. 2013;103(2013):421–9. doi:10.1016/j.fuel.2012.05.054.
Croiset E, Thambimuthu KV. NO x and SO2 emissions from O2/CO2 recycle coal combustion. Fuel. 2001;80(14):2117–21. doi:10.1016/s0016-2361(00)00197-6.
Andersson K, Johnsson F. Process evaluation of an 865 MWe lignite fired O2/CO2 power plant. Energy Convers Manag. 2006;47(18–19):3487–98. doi:10.1016/j.enconman.2005.10.017.
Friedman H. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci C. 1964;6:183–95.
Roduit B. Methodology for lifetime prediction and determination of TMRad and thermal runaway of energetic materials using DSC or C-80 data. Application of thermokinetic concept using DSC, DTA, TG, MS and FTIR signals. Advanced kinetics and technology solutions. Switzerland: AKTS AG; 2006. p. 45.
Kaljuvee T, Keelmann M, Trikkel A, Kuusik R. Thermooxidative decomposition of oil shales. J Therm Anal Cal. 2011;105(2):395–403. doi:10.1007/s10973-010-1033-0.
Advanced Kinetics and Technology Solutions: AKTS thermokinetics. AKTS AG, Switzerland. http://www.akts.com. Accessed 11 Mar 2011.
Acknowledgments
The research was partly financed by Estonian Ministry of Education and Research (target financing SF0140082s08) and by the European Union through the European Regional Development Fund (Projects No. 3.2.0501.10-0002 and 3.2.0501.11-0024).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meriste, T., Yörük, C.R., Trikkel, A. et al. TG–FTIR analysis of oxidation kinetics of some solid fuels under oxy-fuel conditions. J Therm Anal Calorim 114, 483–489 (2013). https://doi.org/10.1007/s10973-013-3063-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3063-x