Abstract
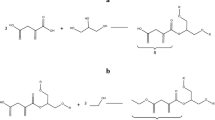
Sugar-based new monomers, polymers, and low molar mass additives have emerged as an exciting topic on green chemistry research, due to the worldwide focus on sustainable material. Isosorbide and its isomers, as “Generally Recognized as Safe” GRAS materials, possess unique stereochemistry and molecular geometry suitable for making cost-effective chemicals and polymers. With growing awareness of bisphenol A (BPA) as a xenoestrogen, isosorbide and its isomers holding the remarkable chemical properties and attractive price can be attached to glycidyl ether to make crosslinkable epoxy resin monomers with similar properties to BPA diglycidyl ether. By adding the hydrophobic functional group into the backbone of isosorbide epoxy or adjusting the amount and type of crosslinker, the mechanical properties and the water uptake ratios (from <1 to >50 wt%) of the isosorbide-derived epoxies could be optimized for different applications. The high water uptake epoxy with controllable biodegradation rate could be used as a drug delivery system or extracellular matrix for biomedical applications while the low water uptake epoxy with strong mechanical properties could be used for can coatings, bone cements, and other industrial additives and adhesives. The chemical structures and properties of the synthesized epoxy monomers and polymers were characterized by DSC, TG, and 1H NMR.



















Similar content being viewed by others
References
Fernando S, Adhikari S, Chandrapal C, Murali N. Biorefineries: current status, challenges, and future direction. Energy Fuel. 2006;20(4):1727–37. doi:10.1021/ef060097w.
Gontard N, Guilbert S. Bio-packaging: technology and properties of edible and/or biodegradable material of agricultural origin. Food packaging and preservation. Berlin: Springer; 1994.
Georgopoulos ST, Tarantili PA, Avgerinos E, Andreopoulos AG, Koukios EG. Thermoplastic polymers reinforced with fibrous agricultural residues. Polym Degrad Stab. 2005;90(2):303–12. doi:10.1016/j.polymdegradstab.2005.02.020.
Narayan R. Polymers from agricultural coproducts. ACS symposium series, vol 575. American Chemical Society; 1994.
Kricheldorf HR. “Sugar Diols” as building blocks of polycondensates. 1997;37(4):599–631.
Mohanty AK, Misra M, Drzal LT. Sustainable bio-composites from renewable resources: opportunities and challenges in the green materials world. J Polym Environ. 2004;10:19–26.
Azapagic A, Emsley A, Hamerton I. Polymers: the environment and sustainable development. West Sussex: Wiley; 2003.
DeSimone LD, Popoff F. Eco-efficiency: the business link to sustainable development. Cambridge: MIT Press; 2000.
Willke T, Vorlop KD. Industrial bioconversion of renewable resources as an alternative to conventional chemistry. Appl Microbiol Biotechnol. 2004;66(2):131–42.
Fukushima K, Kimura Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: their formation, properties, and application. Polym Int. 2006;55(6):626–42.
Wool RP, Sun XS. Bio-based polymers and composites. Boston: Elsevier Academic Press; 2005.
Mohanty AK, Misra M, Hinrichsen G. Biofibres, biodegradable polymers and biocomposites: an overview. Macromol Mater Eng. 2000;276–277(1):1–24.
Thiruvenkatachari R, Ouk Kwon T, Shik Moon I. Application of slurry type photocatalytic oxidation-submerged hollow fiber microfiltration hybrid system for the degradation of bisphenol A (BPA). Sep Sci Technol. 2005;40(14):2871–88. doi:10.1080/01496390500333160.
Haishima Y, Hayashi Y, Yagami T, Nakamura A. Elution of bisphenol-A from hemodialyzers consisting of polycarbonate and polysulfone resins. J Biomed Mater Res. 2001;58(2):209–15. doi:10.1002/1097-4636(2001)58:2<209:aid-jbm1009>3.0.co;2-7.
Yamamoto T, Yasuhara A, Shiraishi H, Nakasugi O. Bisphenol A in hazardous waste landfill leachates. Chemosphere. 2001;42(4):415–8. doi:10.1016/s0045-6535(00)00079-5.
Staples CA, Dome PB, Klecka GM, Oblock ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36(10):2149–73. doi:10.1016/s0045-6535(97)10133-3.
Schafer TE, Lapp CA, Hanes CM, Lewis JB, Wataha JC, Schuster GS. Estrogenicity of bisphenol A and bisphenol A dimethacrylate in vitro. J Biomed Mater Res. 1999;45(3):192–7. doi:10.1002/(sici)1097-4636(19990605)45:3<192:aid-jbm5>3.0.co;2-a.
Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-Fos gene expression in the female reproductive tract. Endocrinology. 1998;139(6):2741–7. doi:10.1210/en.139.6.2741.
Krishnan A, Stathis P, Permuth S, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132(6):2279–86. doi:10.1210/en.132.6.2279.
Feng X, East Anthony J, Hammond W, Jaffe M. Sugar-based chemicals for environmentally sustainable applications. Contemporary science of polymeric materials. ACS symposium series, vol 1061. American Chemical Society; 2010. p. 3–27.
Bengs H, Schoenfeld A, Boehm G, Weis S, Clauss J. Biodegradable polymers, based on natural and renewable raw materials especially isosorbide. US 6,342,300 B1, 2002.
Chatti S, Bortolussi M, Loupy A. Synthesis of New diols derived from dianhydrohexitols ethers under microwave-assisted phase transfer catalysis. Tetrahedron. 2000;56(32):5877–83. doi:10.1016/s0040-4020(00)00539-1.
Chatti S, Bortolussi M, Loupy A. Synthesis of diethers derived from dianhydrohexitols by phase transfer catalysis under microwave. Tetrahedron Lett. 2000;41(18):3367–70. doi:10.1016/s0040-4039(00)00416-0.
Fenouillot F, Rousseau A, Colomines G, Saint-Loup R, Pascault JP. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): a review. Prog Polym Sci. 2010;35(5):578–622. doi:10.1016/j.progpolymsci.2009.10.001.
Feng X, East AJ, Hammond WB, Zhang Y, Jaffe M. Overview of advances in sugar-based polymers. Polym Adv Technol. 2011;22(1):139–50. doi:10.1002/pat.1859.
Xianhong F, Saini P, Vusto G, Hammond WB, East AJ, Jaffe M, editors. Synthesis and thermal analysis of sugar based polyesters as candidate of high performance biopolymers. Bioengineering conference (NEBEC), 2011 IEEE 37th Annual Northeast; 2011, 1–3 April 2011.
Mills JA. The stereochemistry of cyclic derivatives of carbohydrates. In: Melville LW, editor. Advances in carbohydrate chemistry. Amsterdam: Academic Press; 1955. p. 1–53.
Storbeck R, Rehahn M, Ballauff M. Synthesis and properties of high-molecular-weight polyesters based on 1,4:3,6-dianhydrohexitols and terephthalic acid. Die Makromolekulare Chemie. 1993;194(1):53–64.
Storbeck R, Ballauff M. Synthesis and thermal analysis of copolyesters deriving from 1,4:3,6-dianhydrosorbitol, ethylene glycol, and terephthalic acid. J Appl Polym Sci. 1996;59:1196–202.
Xianhong F, Jaffe M, Hammond WB, East AJ, editors. Synthesis of corn-derived carbohydrate derivatives as effective multifunctional sunscreens. Bioengineering Conference, 2009 IEEE 35th Annual Northeast; 2009, 3–5 April 2009.
Cope AC, Shen TY. The stereochemistry of 1,4: 3,6-dianhydrohexitol derivatives 1. J Am Chem Soc. 1956;78(13):3177–82. doi:10.1021/ja01594a055.
Morrison JG. Polyglycidyl ethers of ether anhydro hexitols, method of production, and aqueous solutions thereof. US 3,041,300, 1962.
East A, Laffe M, Zhang Y, Catalani LH. Thermoset epoxy polymers from renewable resources. US 2008/0009599 A1, 2008.
Xianhong F, DeMartino R, East AJ, Hammond WB, Jaffe M, editors. Synthesis and characterization of isosorbide derived polyols as highly effective humectants. Bioengineering conference, Proceedings of the 2010 IEEE 36th Annual Northeast; 2010, 26–28 March 2010.
Acknowledgements
The authors are grateful to the Iowa Corn Promotion Board, USDA and NSF who supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, X., East, A., Hammond, W. et al. Thermal analysis characterization of isosorbide-containing thermosets. J Therm Anal Calorim 109, 1267–1275 (2012). https://doi.org/10.1007/s10973-012-2581-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2581-2