Abstract
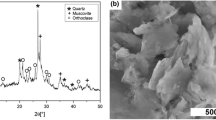
Gels were prepared via sol–gel method by addition of zirconium oxychloride solution into sodium metasilicate (SZ) and sodium metasilicate solution into zirconium oxychloride (ZS) at varying final pH. Si/Zr molar ratio equaled 1/1. Synthesized gels were dried with calcium chloride until they reached a constant mass. SEM and nitrogen adsorption analysis have shown that SZ gels have surface area 175–200 m2 g−1, consist of 20–30 nm grains. ZS samples have surface area about 1 m2 g−1, consist of grains smaller than 10 nm. Thermal and X-ray phase analysis have shown that transition of amorphous ZrO2 to crystalline form shifts from 430 to 850–870 °C for SZ gels. Unlike zirconia gels phase transitions that proceed in order: “amorphous (430 °C)—tetragonal (800 °C)—monoclinic (1,000 °C) phases”, the monoclinic phase in ZS gels appears immediately after transition from amorphous to crystalline state; the tetragonal phase in SZ samples is stable until 1,000 °C.




Similar content being viewed by others
References
Vives S, Meunier C. Influence of the synthesis route on sol–gel SiO2–TiO2 (1:1) xerogels and powders. Ceram Int. 2008;34:37–44.
Bortun A, Bortun M, Pardini J, Khainakov SA, Garcia JR. Synthesis and characterization of mesoporous hydrous zirconium oxide used for arsenic removal from drinking water. Mater Res Bull. 2010;45:142–8.
Zhao Y, Xu L, Wang Y, Gao C, Liu D. Preparation of Ti–Si mixed oxides by sol-gel one-step hydrolysis. Catal Today. 2004;93–95:583–8.
Perez-Hernandez R, Mendoza-Anaya R, Fernandez ME, Gomez-Cortes A. Synthesis of mixed ZrO2–TiO2 oxides by sol-gel: microstructural characterization and infrared spectroscopy studies of NOx. J Mol Catal A. 2008;281:200–6.
Vol’khin VV, Zharnyl’skaya AL, Leont’eva GV. Physicochemical study of composite gel in the Al2O3–ZrO2 system. Russ J Inorg Chem. 2010;5:670–5.
El-Naggar IM, Mowafy EA, El-Aryan YF, Abd El-Wahed MF. Sorption mechanism for Cs+, Co2+ and Eu3+ on amorphous zirconium silicate as cation exchanger. Solid State Ion. 2007;178:741–7.
Landau MV. Sol-gel processing. In: de Jong Krijn P, editor. Synthesis of solid catalysis. Weinheim: Wiley; 2009. p. 83–110.
Aguilar DH, Torres-Gonzalez LC, Torres-Martinez LM, Lopez T, Quintana P. A study of crystallization of ZrO2 in the sol–gel system: ZrO2–SiO2. J Solid State Chem. 2000;158:349–57.
Lucarelli C, Moggi P, Cavani F, Devillers M. Sol-gel synthesis and characterization of transition metal based mixed oxides and their application as catalysts in selective oxidation of propane. App Catal A-Gen. 2007;325:244–50.
Avdin VV, Lymar AA, Batist AV, Nikitin EA, Belkanova MYu, Potemkin VA. Structure formation in heavy metal oxyhydrates at low rates of gel formation. J Struct Chem. 2007;48:796–801.
Avdin VV, Nikitin EA, Lymar AA, Batist AV. Effect of hydrolysis rate on the structure and properties of zirconium oxyhydrates. J Struct Chem. 2009;50:809–16.
Avdin VV, Krivtsov IV, Matveychuk YuV. Sorption properties of mixed zirconium oxyhydrate and silicic acid gels prepared with different mixing methods. South Ural State University Bull Chem. 2010;31:66–71.
da Silva MLCP, Miguel IM, da Silva GLJP. Thermal characterization of hydrous tungsten oxides and their behavior as ion exchanger. J Therm Anal Calorim. 2003;71:493–500.
Ferragina C, Cafarelli F, Di Rocco R. Incorporation of cadmium ions and cadmium sulfide particles into sol-gel zirconium phosphate. Synthesis, thermal behavior and X-ray characterization. J Therm Anal Calorim. 2001;63:709–21.
Szczygiel I, Matraszek A, Checmanowski J, Szczygiel B. Thermal behavior of mixed alumina–silica gels obtained from alkoxides: phase formation and morphology of powders. J Non-Cryst Solids. 2010;36:2824–30.
Sato T. The thermal decomposition of zirconium oxyhydroxide. J Therm Anal Calorim. 2002;69:255–65.
Kostrikin AV, Kosenkova OV, Spiridonov FM, Komissarova LN, Lin’ko IV, Zaitsev BE. On the structure and dehydration of hydrous zirconia and hafnia xerogels. Russ J Inorg Chem. 2010;6:866–75.
Guo Y-G, Chen Y-L, Ying W-L. Thermal, spectroscopic and X-ray diffractional analyses of zirconium hydroxides precipitated at low pH values. Mater Chem Phys. 2004;84:308–14.
Iler RK. The chemistry of silica. New York: Wiley; 1979.
Matveychuk YV, Lymar AA, Avdin VV. Quantum chemical investigation of polymerization in silica gel and copolymerizationin ortho-silicic acid gels with yttrium and lanthanum hydroxides. South Ural State University Bull Chem. 2009;12:42–8.
Kongwudthiti S, Praserthdam P, Tanakulrungsank W, Inoue M. The influence of Si–O–Zr bonds on the crystal-growth inhibition of zirconia prepared by the glycothermal method. J Mater Proc Tech. 2003;136:186–9.
Wu ZG, Zhao YX, Liu DS. The synthesis and characterization of mesoporous silica–zirconia aerogels. Micropor Mesopor Mat. 2004;68:127–32.
Tarafdar A, Pramanik P. Synthesis of amino-functionalized mesoporous silica-zirconia mixed oxide using sodium silicate and zirconium carbonate complex. Micropor Mesopor Mat. 2006;91:221–4.
Acknowledgements
The study is carried out within the Federal Goal-oriented Programme “Scientific and scientific pedagogical personnel of innovative Russia” for 2009–2013, gov.contract. No. 16.740.11.0332.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avdin, V.V., Krivtsov, I.V., Dyachuk, V.V. et al. Thermal behavior of the composite xerogels of zirconium oxyhydroxide and silicic acid. J Therm Anal Calorim 109, 1261–1265 (2012). https://doi.org/10.1007/s10973-012-2531-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2531-z