Abstract
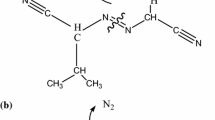
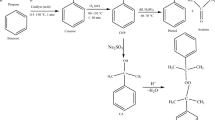
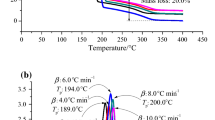
The decomposition of organic peroxides by their relatively weak oxygen linkage and hydroperoxide radical in the presence of reaction solution is one of the thermal hazards for triggering a runaway reaction. Runaway incidents may occur in oxidation reactors, vacuum condensation reactors, tank lorries, or storage tanks. In NFPA 432 organic peroxides in NFPA 432 are classified as flammable. The exothermic behaviors of solid organic peroxides, dicumene peroxide, benzoyl peroxide, and lauroyl peroxide, were determined by differential scanning calorimetry (DSC), and vent sizing package 2 (VSP2). Relevant data detected by DSC provided thermal stability information, such as exothermic onset temperature (T 0), maximum heat-releasing peak (T max), and heat of decomposition (ΔH d). VSP2 was used to perform the bench scale situation for pushing the expected or unexpected reaction to undergo runaway reaction. Onset temperature, maximum pressure, self-heating rate ((dT dt −1)max), and pressure-release rate ((dP dt −1)max) were therefore obtained and explained. These results are essentially crucial in process design for an inherently safer approach.











Similar content being viewed by others
References
Lin CP, Tseng JM, Chang YM, Liu SH, Cheng YC, Shu CM. Modeling liquid thermal explosion reactor containing tert-butyl peroxybenzoate. J Therm Anal Calorim. 2010;102:587–95.
Hou HY, Duh YS, Lee WL, Shu CM. Hazard evaluation for redox system of cumene hydroperoxide mixed with inorganic alkaline solutions. J Therm Anal Calorim. 2009;2:541–5.
Wu LK, Chen KY, Cheng SY, Lee BS, Shu CM. Thermal decomposition of hydrogen peroxide in the presence of sulfuric acid. J Therm Anal Calorim. 2008;93:115–20.
Liu SH, Lin CP, Shu CM. Thermokinetic parameters and thermal hazard evaluation for three organic peroxides by DSC and TAM III. J Therm Anal Calorim. 2011;106:165–72.
Hou HY, Shu CM, Tasi TL. Reactions of cumene hydroperoxide mixed with sodium hydroxide. J Hazard Mater. 2008;152:1214–9.
Chen YL, Chou YP, Hou HY, I YP, Shu CM. Reaction hazard analysis for cumene hydroperoxide with sodium hydroxide or sulfuric acid. J Therm Anal Calorim. 2009;95:535–9.
Bevington JC, Hunt BJ. The use of stabilized radicals with monomers and lauroyl peroxide. Eur Polym J. 2004;40(1):103–8.
You ML, Liu MY, Wu SH, Chi JH, Shu CM. Thermal explosion and runaway reaction simulation of lauroyl peroxide by DSC tests. J Therm Anal Calorim. 2009;96:777–82.
Lu KT, Chen TC, Hu KH. Investigation of the decomposition reaction and dust explosion characteristics of crystalline. J Hazard Mater. 2009;161:246–56.
Zaman F, Beezer AE, Mitchell JC, Clarkson Q, Elliot J, Davis AF, Willson RJ. The stability of benzoyl peroxide by isothermal microcalorimetry. Int J Pharm. 2001;277:133–7.
Somma ID, Marotta R, Andreozzi R, Caprio V. Kinetic and chemical characterization of thermal decomposition of dicumyl peroxide in cumene. J Hazard Mater. 2011;187:157–63.
Wu KW, Hou HY, Shu CM. Thermal phenomena studies for dicumyl peroxide at various concentrations by DSC. J Therm Anal Calorim. 2006;83:41–4.
Shen SJ, Wu SH, Chi JH, Wang YW, Shu CM. Thermal explosion simulation and incompatible reaction of dicumyl peroxide by calorimetric technique. J Therm Anal Calorim. 2010;102:569–77.
Organic peroxide product bulletin. France. Philadelphia, PA: Atofina Chemical Inc.; 2000.
Ando T, Fujimoto T, Morisaki S. Analysis of differential scanning calorimetric data for reactive chemicals. J Hazard Mater. 1991;28:251–80.
Maria G, Heinzle E. Kinetic system identification by using shortcut techniques in early safety assessment of chemical processes. J Loss Prev Proc Ind. 1998;11:187–206.
STARe Software with Solaris Operating System. Operating Instructions. Toledo: Mettler Toledo; 2004.
Wang YW, Duh YS, Shu CM. Evaluation of adiabatic runaway reaction and vent sizing for emergency relief from DSC calorimetry. Ind Eng Chem Res. 2001;40:1125–32.
Huang CC, Peng JJ, Wu SH, Hou HY, You ML, Shu CM. Effects of cumene hydroperoxide of phenol and acetone manufacturing by DSC and VSP2. J Therm Anal Calorim. 2010;102:579–85.
Chang RH, Tseng JM, Jehng JM, Shu CM, Hou HY. Thermokinetic model simulation for methyl ethyl ketone peroxide contaminated with H2SO4 or NaOH by DSC and VSP2. J Therm Anal Calorim. 2006;83:57–62.
Leung JC, Fauske HK, Fisher HG. Thermal runaway reactions in a low thermal inertia apparatus. Thermochim Acta. 1986;104:13–29.
Chervin S, Bodman GT. Method for estimating decomposition characteristics of energetic chemicals. Proc Saf Prog. 2003;22:241–3.
Wu SH, Shyu ML, Chi Y, Shu JC. Evaluation of runaway reaction for dicumyl peroxide in a batch reactor by DSC and VSP2. J Loss Prev Process Ind. 2009;22:721–7.
Acknowledgements
The authors are indebted to the donors of the National Science Council (NSC) in Taiwan under the contract number NSC-99-2221-E-224-029-MY3 for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, LC., Chen, JW., Hou, HY. et al. Exothermic behaviors in decomposition of three solid organic peroxides by DSC and VSP2. J Therm Anal Calorim 109, 1303–1309 (2012). https://doi.org/10.1007/s10973-012-2520-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2520-2