Abstract
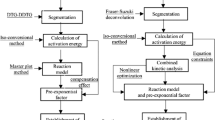
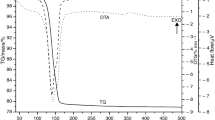
Practical usefulness of the kinetic deconvolution for partially overlapped thermal decomposition processes of solids was examined by applying to the co-precipitated basic zinc carbonate and zinc carbonate. Comparing with the experimental deconvolutions by thermoanalytical techniques and mathematical deconvolutions using different statistical fitting functions, performance of the kinetic deconvolution based on an accumulative kinetic equation for the independent processes overlapped partially was evaluated in views of the peak deconvolution and kinetic evaluation. Two-independent kinetic processes of thermal decompositions of basic zinc carbonate and zinc carbonate were successfully deconvoluted by means of the thermoanalytical measurements in flowing CO2 and by applying sample controlled thermal analysis (SCTA). The deconvolutions by the mathematical curve fittings using different fitting functions and subsequent formal kinetic analysis provide acceptable values of the mass-loss fractions and apparent activation energies of the respective reaction processes, but the estimated kinetic model function changes depending on the fitting functions employed for the peak deconvolution. The mass-loss fractions and apparent kinetic parameters of the respective reaction processes can be optimized simultaneously by the kinetic deconvolution based on the kinetic equation through nonlinear least square analysis, where all the parameters indicated acceptable correspondences to those estimated through the experimental and mathematical deconvolutions. As long as the reaction processes overlapped are independent kinetically, the simple and rapid procedure of kinetic deconvolution is useful as a tool for characterizing the partially overlapped kinetic processes of the thermal decomposition of solids.













Similar content being viewed by others
References
Sorai M, editor. Comprehensive handbook of calorimetry & thermal analysis. Chichester: Wiley; 2004.
Sørensen OT, Rouquerol J, editors. Sample controlled thermal analysis. Dordrecht: Kluwer; 2003.
Sanchez-Jimenez PE, Perez-Maqueda LA, Crespo-Amoros JE, Lopez J, Perejon A, Criado JM. Quantitative characterization of multicomponent polymers by sample-controlled thermal analysis. Anal Chem. 2010;82(21):8875–80. doi:10.1021/ac101651g.
Bernard S, Fiaty K, Cornu D, Miele P, Laurent P. Kinetic modeling of the polymer-derived ceramics route: investigation of the thermal decomposition kinetics of poly[B-(methylamino)borazine] precursors into boron nitride. J Phys Chem B. 2006;110(18):9048–60. doi:10.1021/jp055981m.
Ozao R, Nishimoto Y, Pan W, Okabe T. Thermoanalytical characterization of carbon/carbon hybrid material, apple woodceramics. Thermochim Acta. 2006;440(1):75–80. doi:10.1016/j.tca.2005.10.014.
Cai J, Liu R. Weibull mixture model for modeling nonisothermal kinetics of thermally stimulated solid-state reactions: application to simulated and real kinetic conversion data. J Phys Chem B. 2007;111(36):10681–6. doi:10.1021/jp0737092.
Koga N, Yamane Y. Effect of mechanical grinding on the reaction pathway and kinetics of the thermal decomposition of hydromagnesite. J Therm Anal Calorim. 2008;93(3):963–71. doi:10.1007/s10973-007-8616-4.
Cai J, Alimujiang S. Kinetic analysis of wheat straw oxidative pyrolysis using thermogravimetric analysis: statistical description and isoconversional kinetic analysis. Ind Eng Chem Res. 2009;48(2):619–24. doi:10.1021/ie801299z.
Vecchio S, Cerretani L, Bendini A, Chiavaro E. Thermal decomposition study of monovarietal extra virgin olive oil by simultaneous thermogravimetry/differential scanning calorimetry: relation with chemical composition. J Agric Food Chem. 2009;57(11):4793–800. doi:10.1021/jf900120v.
Janković B, Adnađević B, Kolar-Anić L, Smičiklas I. The non-isothermal thermogravimetric tests of animal bones combustion. Part II. Statistical analysis: application of the Weibull mixture model. Thermochim Acta. 2010;505(1–2):98–105. doi:10.1016/j.tca.2010.04.005.
Perejon A, Sanchez-Jimenez PE, Criado JM, Perez-Maqueda LA. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B. 2011;115(8):1780–91. doi:10.1021/jp110895z.
Mamleev V, Bourbigot S, Le Bras M, Duquesne S, Šesták J. Thermogravimetric analysis of multistage decomposition of materials. Phys Chem Chem Phys. 2000;2(20):4796–803. doi:10.1039/b004357p.
Ferriol M, Gentilhomme A, Cochez M, Oget N, Mieloszynski JL. Thermal degradation of poly(methyl methacrylate) (PMMA): modelling of DTG and TG curves. Polym Degrad Stab. 2003;79(2):271–81. doi:10.1016/S0141-3910(02)00291-4.
Font R, Conesa JA, Moltó J, Muñoz M. Kinetics of pyrolysis and combustion of pine needles and cones. J Anal Appl Pyrol. 2009;85(1–2):276–86. doi:10.1016/j.jaap.2008.11.015.
Lopez G, Aguado R, Olazar M, Arabiourrutia M, Bilbao J. Kinetics of scrap tyre pyrolysis under vacuum conditions. Waste Manag. 2009;29(10):2649–55. doi:10.1016/j.wasman.2009.06.005.
Sánchez-Jiménez PE, Perejón A, Criado JM, Diánez MJ, Pérez-Maqueda LA. Kinetic model for thermal dehydrochlorination of poly(vinyl chloride). Polymer. 2010;51(17):3998–4007. doi:10.1016/j.polymer.2010.06.020.
Koga N, Sestak J, Malek J. Distortion of the Arrhenius parameters by the inappropriate kinetic-model function. Thermochim Acta. 1991;188(2):333–6. doi:10.1016/0040-6031(91)87091-a.
Koga N. A review of the mutual dependence of Arrhenius parameters evaluated by the thermoanalytical study of solid-state reactions: the kinetic compensation effect. Thermochim Acta. 1994;244(1):1–20. doi:10.1016/0040-6031(94)80202-5.
Koga N, Criado JM, Tanaka H. Kinetic analysis of the thermal decomposition of synthetic malachite by CRTA. J Therm Anal Calorim. 2000;60(3):943–54. doi:10.1023/a:1010172111319.
Koga N, Yamada S. Influences of product gases on the kinetics of thermal decomposition of synthetic malachite evaluated by controlled rate evolved gas analysis coupled with thermogravimetry. Int J Chem Kinet. 2005;37(6):346–54. doi:10.1002/kin.20089.
Alcalá M, Criado JM, Gotor FJ, Ortega A, Perez Maqueda LA, Real C. Development of a new thermogravimetric system for performing constant rate thermal analysis (CRTA) under controlled atmosphere at pressures ranging from vacuum to 1 bar. Thermochim Acta. 1994;240(1):167–73. doi:10.1016/0040-6031(94)87038-1.
Koga N, Criado JM. The influence of mass transfer phenomena on the kinetic analysis for the thermal decomposition of calcium carbonate by constant rate thermal analysis (CRTA) under vacuum. Int J Chem Kinet. 1998;30(10):737–44. doi:10.1002/(sici)1097-4601(1998)30:10<737:aid-kin6>3.0.co;2-w.
Perez-Maqueda LA, Criado JM, Gotor FJ. Controlled rate thermal analysis commanded by mass spectrometry for studying the kinetics of thermal decomposition of very stable solids. Int J Chem Kinet. 2002;34(3):184–92. doi:10.1002/kin.10042.
Criado JM, Pérez-Maqueda LA, Diánez MJ, Sánchez-Jiménez PE. Development of a universal constant rate thermal analysis system for being used with any thermoanalytical instrument. J Therm Anal Calorim. 2007;87(1):297–300. doi:10.1007/s10973-006-7813-x.
Kanari N, Mishra D, Gaballah I, Dupre B. Thermal decomposition of zinc carbonate hydroxide. Thermochim Acta. 2004;410(1–2):93–100. doi:10.1016/S0040-6031(03)00396-4.
Koga N, Tanaka H. Thermal decomposition of Copper(II) and zinc carbonate hydroxides by means of TG-MS—quantitative analyses of evolved gases. J Therm Anal and Calorim. 2005;82(3):725–9. doi:10.1007/s10973-005-0956-3.
Hales MC, Frost RL. Synthesis and vibrational spectroscopic characterisation of synthetic hydrozincite and smithsonite. Polyhedron. 2007;26(17):4955–62. doi:10.1016/j.poly.2007.07.002.
Gotor FJ, Macías M, Ortega A, Criado JM. Simultaneous use of isothermal, nonisothermal, and constant rate thermal analysis (CRTA) for discerning the kinetics of the thermal dissociation of smithsonite. Int J Chem Kinet. 1998;30(9):647–55. doi:10.1002/(SICI)1097-4601(1998)30:9<647:AID-KIN6>3.0.CO;2-S.
Budrugeac P, Criado JM, Gotor FJ, Popescu C, Segal E. Kinetic analysis of dissociation of smithsonite from a set of non-isothermal data obtained at different heating rates. J Therm Anal Calorim. 2001;63(3):777–86. doi:10.1023/A:1010148306206.
Vágvölgyi V, Hales M, Martens W, Kristóf J, Horváth E, Frost RL. Dynamic and controlled rate thermal analysis of hydrozincite and smithsonite. J Therm Anal Calorim. 2008;92(3):911–6.
Hales MC, Frost RL. Thermal analysis of smithsonite and hydrozincite. J Therm Anal Calorim. 2008;91(3):855–60. doi:10.1007/s10973-007-8571-0.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Fraser RDB, Suzuki E. Resolution of overlapping absorption bands by least squares procedures. Anal Chem. 1966;38(12):1770–3. doi:10.1021/ac60244a038.
Fraser RDB, Suzuki E. Resolution of overlapping bands. Functions for simulating band shapes. Anal Chem. 1969;41(1):37–9. doi:10.1021/ac60270a007.
Koga N, Tatsuoka T, Tanaka Y, Yamada S. Catalytic action of atmospheric water vapor on the thermal decomposition of synthetic hydrozincite. Trans Mater Res Soc Jpn. 2009;34(2):343–6.
Yamada S, Tsukumo E, Koga N. Influences of evolved gases on the thermal decomposition of zinc carbonate hydroxide evaluated by controlled rate evolved gas analysis coupled With TG. J Therm Anal Calorim. 2009;95(2):489–93. doi:10.1007/s10973-008-9272-z.
Friedman HL. Kinetics of thermal degradation of cha-forming plastics from thermogravimetry, application to a phenolic plastic. J Polym Sci C. 1964;6:183–95.
Criado J, Ortega A. Non-isothermal transformation kinetics: remarks on the Kissinger method. J Non-Cryst Solids. 1986;87(3):302–11. doi:10.1016/s0022-3093(86)80004-7.
Budrugeac P, Segal E. Applicability of the Kissinger equation in thermal analysis. J Therm Anal Calorim. 2007;88(3):703–7. doi:10.1007/s10973-006-8087-z.
Ozawa T. Applicability of Friedman plot. J Therm Anal. 1986;31:547–51.
Koga N. Kinetic-analysis of thermoanalytical data by extrapolating to infinite temperature. Thermochim Acta. 1995;258:145–59. doi:10.1016/0040-6031(95)02249-2.
Gotor FJ, Criado JM, Malek J, Koga N. Kinetic analysis of solid-state reactions: the universality of master plots for analyzing isothermal and nonisothermal experiments. J Phys Chem A. 2000;104(46):10777–82. doi:10.1021/jp0022205.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6. doi:10.1246/bcsj.38.1881.
Ozawa T. Non-isothermal kinetics and generalized time. Thermochim Acta. 1986;100(1):109–18. doi:10.1016/0040-6031(86)87053-8.
Sestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12. doi:10.1016/0040-6031(71)85051-7.
Šesták J. Diagnostic limits of phenomenological kinetic models introducing the accommodation function. J Therm Anal. 1990;36(6):1997–2007. doi:10.1007/bf01914116.
Málek J. The kinetic analysis of non-isothermal data. Thermochim Acta. 1992;200:257–69. doi:10.1016/0040-6031(92)85118-f.
Perez-Maqueda LA, Criado JM, Sanchez-Jimenez PE. Combined kinetic analysis of solid-state reactions: a powerful tool for the simultaneous determination of kinetic parameters and the kinetic model without previous assumptions on the reaction mechanism. J Phys Chem A. 2006;110(45):12456–62. doi:10.1021/jp064792g.
Šimon P. Fourty years of the Šesták–Berggren equation. Thermochim Acta. 2011;520(1–2):156–7. doi:10.1016/j.tca.2011.03.030.
Ozao R, Ochiai M. Fractal reaction in solids. J Ceram Soc Jpn. 1993;101(11):263–7. doi:10.2109/jcersj.101.263.
Koga N. Accommodation of the actual solid-state process in the kinetic-model function. 1. Significance of the nonintegral kinetic exponents. J Therm Anal. 1994;41(2–3):455–69. doi:10.1007/bf02549327.
Koga N, Malek J. Accommodation of the actual solid-state process in the kinetic model function. 2. Applicability of the empirical kinetic model function to diffusion-controlled reactions. Thermochim Acta. 1996;283:69–80.
Koga N, Criado JM. Kinetic analyses of solid-state reactions with a particle-size distribution. J Amer Ceram Soc. 1998;81(11):2901–9. doi:10.1111/j.1151-2916.
Koga N, Tanaka H. A physico-geometric approach to the kinetics of solid-state reactions as exemplified by the thermal dehydration and decomposition of inorganic solids. Thermochim Acta. 2002;388(1–2):41–61. doi:10.1016/s0040-6031(02)00051-5.
de Levie R. Collinearity in least-squares analysis. J Chem Educ. 2012;89(1):68–78. doi:10.1021/ed100947d.
de Levie R. Nonisothermal analysis of solution kinetics by spreadsheet simulation. J Chem Educ. 2012;89(1):79–86. doi:10.1021/ed100948n.
Khawam A, Flanagan DR. Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B. 2006;110(35):17315–28. doi:10.1021/jp062746a.
Acknowledgements
This study was supported partially by the grant-in-aid for scientific research (B) (21360340 and 22300272) from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koga, N., Goshi, Y., Yamada, S. et al. Kinetic approach to partially overlapped thermal decomposition processes. J Therm Anal Calorim 111, 1463–1474 (2013). https://doi.org/10.1007/s10973-012-2500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2500-6