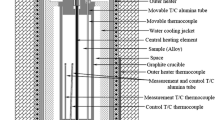
Abstract
The results of calorimetric study and thermal analysis of binary Al–Sn system are presented in this paper. The Oelsen calorimetry was used in thermodynamic analysis. Following thermodynamic properties were determined at 727 °C: activities, activity coefficients, partial/integral molar Gibbs excess, and mixing energies. The energetics of mixing in liquid Al–Sn alloys has been analyzed through the study of concentration fluctuation in the long-wavelength limit. Thermal analysis of selected alloys in Al–Sn system was done using differential thermal analysis. Defined characteristic phase transition temperatures were used for comparison with calculated phase diagram of investigated system. Good agreement with available literature data was obtained. Structural analysis of selected alloys was done using optic microscopy.






Similar content being viewed by others
References
Yuan G, Zhang X, Lou Y, Li Z. Tribological characteristics of new series of Al–Sn–Si alloys. Trans Nonferrous Met Soc China. 2003;13(4):774–80.
Abis S, Barucca G, Mengucci P. Electron microscope characterization of Al–Sn metal–metal matric composites. J Alloy Compd. 1994;215(11):309–14.
Abis S, Onofrio G. New bearing aluminum-based alloys. In: proceedings of advanced materials, Milano (Italy); 1989. pp. 511–513.
Schouwenaars R, Torres JA, Jacobo VH, Ortiz A. Tailoring the mechanical properties of Al–Sn alloys for tribological applications. Mater Sci Forum. 2007;317:539–43.
Keijiti I, Zoshimi M, Kenichiro F. (Toyota, Japan): Al–Sn base bearing alloy and composite, United States Patent 4296183 (http://www.freepatentsonline.com/4296183.html).
Kitajima M, Shono T. Development of Sn–Zn–Al lead-free solder alloys. Fujitsu Sci Technol J. 2005;41(2):225–35.
Apelian D. Aluminum cast alloys: enabling tools for improved performance. North American Die Casting Association. Wheeling, Illinois; 2009.
McAlister AJ. In: Massalski TB, editor. Binary alloy phase diagrams. Metals Park: Am Soc Met; 1986.
Hultgren R, Desai PD, Hawkins DT, Gleiser M, Kelley KK. Selected values of the thermodynamic properties of binary alloys. Metal Parks: ASM; 1973.
Prasad LC, Mikula A. Thermodynamics of liquid Al–Sn–Zn alloys and concerned binaries in the light of soldering characteristics. Phys B. 2006;373:64–71.
Yazawa A, Lee YK. Thermodynamic studies of the liquid aluminum alloy systems. Trans Jpn Inst Metal. 1970;11(6):411–8.
Popescu CA, Taloi D. Thermodynamic calculation in liquid Al-Sn alloys systems. UPB Sci Bull Ser B. 2007;69(3):77–84.
Dinsdale AT, Kroupa A, Vizdal J, Vreštal J, Watson A, Zemanova A. COST 531 Database for Lead-free Solders Ver 20. 2006.
Bonnier E, Durand F, Massart G. Thermodynamic study of the system AI-Sn. CR Acad Sci Paris. 1964;259:380–3.
Massart G, Durand F, Bonnier E. Thermodynamic properties of liquid AI-Sn alloys via emf studies. Bull Soc Chim France. 1965;1:87–90.
Oelsen W, Zühlke P, Oelsen O. Kalorimetrie und thermodynamic der Al-Sn Legierungen. Arch Eisenhütenwesen. 1958;29:799–804.
Wittig FE, Keil G. The heat of mixing of Al with Zn, Cd, In, Th, Sn, Pb, and Bi. Z Metallkd. 1963;54:576–90.
Oelsen W, Tebbe W, Oelsen O. Zur thermodynamischen Analyse VII—Das Trockneis-Kalorimeter. Arch Eisenhuttenwess. 1956;27:689–94.
Oelsen W, Schurmann E, Weigt HJ, Oelsen O. Zur thermodynamischen Analyse IV—Vermischungsentropie und Bildungsaffinitat der Blei-Kadmium-Schmelzen aus kalorimetrischen Messungen. Arch Eisenhuttenwess. 1956;27:487–511.
Oelsen W, Bieret F, Schwabe G. Zur thermodynamischen Analyse VI—Kalorimetrie und Thermodynamic der Wismut-Kadmium-Legierungen. Arch Eisenhuttenwess. 1956;27:607–20.
Živković D, Katayama I, Gomidželović L, Manasijević D, Novaković R. Comparative thermodynamic study and phase equilibria of the Bi–Ga–Sn ternary system. Int J Mater Res. 2007;98(10):1025–30.
Živković D, Mitovski A, Balanović Lj, Manasijević D, Živković Ž. Thermodynamic analysis of liquid In–Sn alloys using Oelsen calorimetry. J Therm Anal Calorim. 2010;102(3):827–30.
Singh RN. Short range order and concentration fluctuations in binary molten alloys, (Special issue—liquid metals & alloys). Can J Phys. 1987;65:309–25.
Akinlade O, Boyo AO, Ijaduola BR. Demixing tendencies in some Sn-based liquid alloys. J Alloy Compd. 1999;290:191–6.
COST MP0602 Thermodynamic Database Version 10. 2009.
Acknowledgements
The authors are grateful for support to the Ministry of Education and Science of the Republic of Serbia (Project No 172037).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balanović, L., Živković, D., Manasijević, D. et al. Calorimetric study and thermal analysis of Al–Sn system. J Therm Anal Calorim 111, 1431–1435 (2013). https://doi.org/10.1007/s10973-012-2499-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2499-8