Abstract
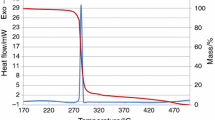
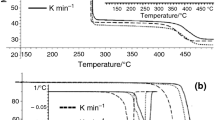
Thermal behavior and decomposition kinetics of Formex-bonded PBXs based on some attractive cyclic nitramines, such as 1,3,5-trinitro-1,3,5-triazinane (RDX) and 1,3,5,7-tetranitro-1,3,5,7-tetrazocane (HMX). Actually, cis-1,3,4,6-tetranitrooctahy droimidazo-[4,5-d]imidazole (BCHMX) and 2,4,6,8,10,12-hexanitro-2,4,6,8,10, 12-hexaazaisowurtzitane (CL-20), was investigated by means of nonisothermal thermogravimetry (TG) and differential scanning calorimetry (DSC). It was found that the mass loss rate of PBXs involved in this research depends greatly on heating rate and the residue of the decomposition of these PBXs decreases with the heating rate. The onset of the exotherms was noticed at 215.4, 278.7, 231.2 and 233.7 °C with the peak maximum at 235.1, 279.0, 231.2 and 233.7 °C for RDX-Formex, HMX-Formex, CL-20-Formex, and BCHMX-Formex, respectively. Their corresponding exothermic changes were 1788, 1237, 691, and 1583 J g−1. It was also observed that the dependence on the heating rate for onset temperatures of HMX- and BCHMX-based PBXs was almost the same due to their similar molecular structure. In addition, based on nonisothermal TG data, the kinetic parameters for thermal decomposition of these PBXs were calculated by isoconversional methods. It was shown that the Formex base has great effects on the activation energy distribution of nitramines. It was further found that the kinetic compensation effects occurred during the thermal decomposition of nitramine-based PBXs, and they almost have the same compensation effects due to similar decomposition mechanism.









Similar content being viewed by others
References
Clements BE, Mas EM. A theory for plastic-bonded materials with a bimodal size distribution of filler particles. Modell Simul Mater Sci Eng. 2004;12(3):407–21.
Nouguez B, Mahé B, Vignaud PO. Cast PBX related technologies for IM shells and warheads. Sci Technol Energ Mater. 2009;70(5–6):135–9.
Chapman RD, Wilson WS, Fronabarger JW, Merwin LH, Ostrom GS. Prospects of fused polycyclic nitroazines as thermally insensitive energetic materials. Thermochim Acta. 2002;384(1–2):229–43.
Elbeih A, Pachman J, Zeman S, Akštein Z. Replacement of PETN by Bicyclo-HMX Semtex 10. In: 8th international armament conference on scientific aspects of armament and safety technology, Pułtusk; 2010. p. 7–16.
Elbeih A, Pachman J, Zeman S, Trzciński W, Akštein Z. Advanced plastic explosive based on BCHMX compared with composition C4 and Semtex 10. In: new trends in research of energetic materials, Czech Republic; 2011. p. 119–126.
Klasovitý D, Zeman S. Preparation of cis-1,3,4,6-tetranitrooctahydroimidzo-[4,5-d]imidazole (bicycle-HMX, BCHMX) by two step synthesis. Czech Appl. Pat. PV-2009-503, Int. Cl.: C07D 487/04, University of Pardubice, 28 July 2009.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32.
Yi C. The correctional kinetic equation for the peak temperature in the differential thermal analysis. J Therm Anal Calorim. 2008;93(1):111–3.
Sell T, Vyazovkin S, Wight CA. Thermal decomposition kinetics of PBAN—binder and composite solid rocket propellants, combust. Flame. 1999;119:174.
Felix SP, Singh G, Sikder AK, Aggrawal JP. Studies on energetic compounds—part 33: thermolysis of keto-RDX and its plastic bonded explosives containing thermally stable polymers. Thermochim Acta. 2005;426:53–60.
Gilardi RF, Anderson JL, Evans R. Cis-2,4,6,8-tetranitro-1H,5H-2,4,6,8-tetraazabicyclo [3.3.0] octane, the energetic compound (bicyclo-HMX). Acta Crystallogr. 2002; 58:0972.
Klasovity D, Zeman S, Ruzicka A, Jungova M, Rohac M. cis-1,3,4,6-Tetranitrooctahydroimidazo-[4,5-d]imidazole (BCHMX), its properties and initiation reactivity. J Hazard Mater. 2009;164(2–3):954–61.
Elbeih A, Pachman J, Trzcinski WA, Zeman S, Akstein Z, Šelesovsky J. Study of plastic explosives based on attractive cyclic nitramines Part I. Detonation characteristics of explosives with PIB binder. Propellants Explos Pyrotech. 2011;36(5):433–8.
Burnham AK. A Comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application predictions. In: NATAS 34th annual conference, Bowling Green, 6–9 Aug 2006 UCRL-CONF-221685.
Sánchez-Jiménez PE, Pérez-Maqueda LA, Perejón A, Criado JM. Generalized kinetic master plots for the thermal degradation of polymers following a random scission mechanism. J Phys Chem A. 2010;114(30):7868–76.
Sanchez-Jimenez PE, Criado JM, Perez-Maqueda LA. Kissinger kinetic analysis of data obtained under different heating schedules. J Therm Anal Calorim. 2008;94:427.
Perez-Maqueda LA, Sanchez-Jimenez PE, Criado JM. Kinetic analysis of solid-state reactions: precision of the activation energy calculated by integral methods. Int J Chem Kinet. 2005;37:658.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404(1–2):163–76.
Starink MJ. Activation energy determination for linear heating experiments: deviations due to neglecting the low temperature end of the temperature integral. J Mater Sci. 2007;424(2):83–489.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Atkins P, de Paula J. Physical chemistry (discussion of negative activation energies is also found in earlier editions, see subject index under “activation energy, negative”). 9th ed. New York: W.H. Freeman; 2010.
Brown ME. Introduction to thermal analysis. 2nd ed. Dodrecht: Kluwer; 2001.
Dong X-F, Yan Q-L, Zhang X-H. Effect of potassium chlorate on thermal decomposition of cyclotrimethylenetrinitramine (RDX). J Anal Appl Pyrol. 2012;93:160–4.
Liao L-Q, Yan Q-L, Zheng Y. Thermal decomposition mechanism of particulate core-shell KClO3-HMX composite energetic material. Indian J Eng Mater Sci. 2011;18(5):393–8.
Yan Q-L, Song Z-W, Shi X-B, Yang Z-Y, Zhang X-H. Combustion mechanism of double-base propellant containing nitrogen heterocyclic nitroamines (II): the temperature distribution of the flame and its chemical structure. Acta Astronaut. 2009;64(5–6):602–14.
Elbeih A, Zeman S, Jungová M, Akštein Z, Vávra P. Detonation characteristics and penetration performance of plastic explosives. In: Li S, Niu P, editors. Theory and practice of energetic materials, vol. IX. Beijing: Science Press; 2011. p. 508–13.
Zeman S. Sensitivities of high energy compounds. In: Klapoetke T, editor. High energy density materials, series: structure and bonding, 125. New York: Springer; 2007. p. 195–271.
Kim D-Y, Kim K-J. Solubility of cyclotrimethylenetrinitramine (RDX) in binary solvent mixtures. J Chem Eng Data. 2007;52(5):1946–9.
Shao Y-H, Ren X-N, Liu Z-R. An investigation on eutectic binary phase diagram of volatilizable energetic materials by high pressure DSC. J Therm Anal Calorim. 2011;101(3):1135–41.
Colomba DB. Analysis of convection and secondary reaction effects within porous solid fuels undergoing pyrolysis. Combust Sci Technol. 1993;90(5–6):315–40.
Tarver CM, Tran TD. Thermal decomposition models for HMX-based plastic bonded explosives. Combust Flame. 2004;137(1–2):50–62.
Singh G, Felix SP, Soni P. Studies on energetic compounds part 28: thermolysis of HMX and its plastic bonded explosives containing Estane. Thermochim Acta. 2003;399(1–2):153–65.
Singh G, Felix SP, Soni P. Studies on energetic compounds part 31: thermolysis and kinetics of RDX and some of its plastic bonded explosives. Thermochim Acta. 2005;426:131–9.
Lee J, Jaw K-S. Thermal decomposition properties and compatibility of CL-20, NTO with silicone rubber. J Therm Anal Calorim. 2006;85(2):463–7.
Ninan KN, Catherine KB, Krishnan K. Effect of molecular weight on non-isothermal decomposition kinetics of hydroxyl terminated polybutadiene. J Therm Anal Calorim. 1990;36(3):855–67.
Barrie PJ. The mathematical origins of the kinetic compensation effect: 1. the effect of random experimental errors. Phys Chem Chem Phys. 2012;14:318–26.
Acknowledgements
The work in this paper was mainly carried out as a part of the Ministry of Interior of the Czech Republic Project No. VG20102014032. The work was also partially supported by the research project (No. MSM 00221627501) provided by the Ministry of Education, Youth & Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, QL., Zeman, S., Šelešovský, J. et al. Thermal behavior and decomposition kinetics of Formex-bonded explosives containing different cyclic nitramines. J Therm Anal Calorim 111, 1419–1430 (2013). https://doi.org/10.1007/s10973-012-2492-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2492-2