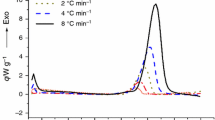
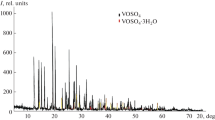
Abstract
The results of investigations on thermal decomposition of NH4VO3 in dry air have been presented. TG–DSC measurements were carried out under non-isothermal conditions at linear change of samples temperature in time and under isothermal conditions. Characterization of the products structure was performed by XRD method. MS method was used to determine evolved gaseous products. The decomposition of NH4VO3 was described by the following equation:










Similar content being viewed by others
Abbreviations
- α:
-
Conversion degree
- β:
-
Heating rate/K/min
- m 0 :
-
Initial sample mass for the stage/mg
- m :
-
Current sample mass for the stage/mg
- m k :
-
Final sample mass after for the stage/mg
- t :
-
Time/min
- T :
-
Temperature/K
- T o :
-
Initial temperature of the stage/K
- T m :
-
Temperature corresponding to peak maximum/K
- TGu :
-
Normalized TG
References
Rogl P, Bittermann H. Ternary metal boron carbides. Int Refract J Hard Mater. 1999;17:27–32.
Ye J, Liu Y, Zhao Z, Jiang Z, Tang Z. Synthesis of VC nanopowders by thermal processing of precursor with CaF2 addition. J Alloy Comp. 2010;496(1–2):278–81.
Liu YB, Liu Y, Tang HP, Wang B, Liu B. Reactive sintering mechanism of Ti + Mo2C and Ti + VC powder compacts. J Mater Sci. 2011;46(4):902–9.
Jin Y, Liu Y, Wang Y, Ye J. Synthesis of (Ti, W, Mo, V) (C, N) nonocomposite powder from novel precursors. Int Refract J Hard Mater. 2010;28:541–3.
Mutin PH, Popa AF, Vioux A, Delahay G, Coq B. Nonhydrolytic vanadian–titania xerogels: synthesis, characterization, and behavior in the selective catalytic reduction of NO by NH3. Applied Catal B. 2006;69(1–2):49–57.
Debecker DP, Bouchmella K, Delaigle R, Eloy P, Poleunis C, Bertrand P, Gaigneaux EM, Mutin PH. One-step non-hydrolytic sol–gel preparation of efficient V2O5–TiO2 catalysts for VOC total oxidation. Applied Catal B. 2010;94(1–2):38–45.
Biedunkiewicz A, Strzelczak A, Możdżeń G, Lelątko J. Non-isothermal oxidation of ceramic nanocomposites using the example of Ti–Si–C–N powder: kinetic analysis method. Acta Mater. 2008;56:3132–45.
Biedunkiewicz A, Strzelczak A, Chrościechowska J. Non-isothermal oxidation of TiC x powder in dry air. Pol J Chem Technol. 2005;7(4):1–10.
Biedunkiewicz A, Gordon N, Straszko J, Tamir S. Kinetics of thermal oxidation of titanium carbide and its carbon nano-composites in dry air atmosphere. J Therm Anal Calorim. 2007;88(3):717–22.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Brauner N, Shacham M. Statistical analysis of linear and nonlinear correlation of the Arrhenius equation constants. Chem Eng Proc. 1997;36(3):243–9.
Schwaab M, Pinto JC. Optimum reference temperature for reparameterization of the Arrhenius equation. Part 1: problems involving one kinetic constant. Chem Eng Sci. 2007;62(10):2750–64.
Sbirrazzuoli N, Vincent L, Vyazovkin S. Comparison of several computational procedures for evaluating the kinetics of thermally stimulated condensed phase reactions. Chemometr Intell Lab Syst. 2000;54(1):53–60.
Galway AK. What is meant by the term “variable activation energy” when applied in the kinetic analyses of solid state decompositions (cryptolysis reaction)? Thermochim Acta. 2003;397:249–68.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Wanjun T, Yuwen L, Xi Y, Cunxin W. Kinetic studies of the calcination of ammonium metavanadate by thermal methods. Ing Eng Chem Res. 2004;43(9):2054–9.
Acknowledgements
Financial support of the study by the Ministry of Science and Higher Education within (The National Centre for Research and Development) the project No. NR15-0067-10/2010-2013, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biedunkiewicz, A., Gabriel, U., Figiel, P. et al. Investigations on NH4VO3 thermal decomposition in dry air. J Therm Anal Calorim 108, 965–970 (2012). https://doi.org/10.1007/s10973-011-2149-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2149-6