Abstract
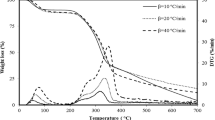
The pyrolytic characteristics and kinetics of wetland plant Phragmites australis was investigated using thermogravimetric method from 50 to 800 °C in an inert argon atmosphere at different heating rates of 5, 10, 25, 30, and 50 °C min−1. The kinetic parameters of activation energy and frequency factor were deduced by appropriate methods. The results showed that three stages appeared in the thermal degradation process. The most probable mechanism functions were described, and the average apparent activation energy was deduced as 291.8 kJ mol−1, and corresponding pre-exponential factors were determined as well. The results suggested that the most probable reaction mechanisms could be described by different models within different temperature ranges. It showed that the apparent activation energies and the corresponding pre-exponential factors could be obtained at different conversion rates. The results suggested that the experimental results and kinetic parameters provided useful information for the design of pyrolytic processing system using P. australis as feedstock.



Similar content being viewed by others
References
Demirbas A. Progress and recent trends in biofuels. Prog Energy Combust Sci. 2007;33:1–18.
Lewandowski I, Scurlock JMO, Lindvall E, Christou M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biom Bioen. 2003;25:335–61.
Basso MC, Cerrella EG, Buonomo EL, Bonelli PR, Cukierman AL. Thermochemical conversion of Arundo donax into useful solid products. Ener Sour. 2005;27:1429–38.
Sujjakulnukit B, Yingyuad R, Maneekhao V, Pongnarintasut V, Bhattacharya SC, Abdul Salam P. Assessment of sustainable energy potential of non-plantation biomass resources in Thailand. Biom Bioen. 2005;29:214–24.
Artigas F, Pechmann IC. Balloon imagery verification of remotely sensed Phragmites australis expansion in an urban estuary of New Jersey. Landsc Urban Plan. 2010;95:105–12.
Bridgwater T. Biomass for energy. J Sci Food Agr. 2006;86:1755–68.
Pevida C, Plaza MG, Arias B, Fermoso J, Rubiera F, Pis JJ. Surface modification of activated carbons for CO2 capture. Appl Surf Sci. 2008;254:7165–72.
Erlich C, Björnbom E, Bolado D, Giner M, Fransson TH. Pyrolysis and gasification of pellets from sugarcane bagasse and wood. Fuel. 2006;85:1535–40.
Isci A, Demirer GN. Biogas production potential from cotton wastes. Renew Energy. 2007;32:750–7.
Lapuerta M, Hernández JJ, Rodríguez J. Comparison between the kinetics of devolatilization of forestry and agricultural wastes from the middle-south regions of Spain. Biom Bioen. 2007;31:13–9.
Park YH, Kim J, Kim SS, Park YK. Pyrolysis characteristics and kinetics of oak trees using thermo- gravimetric analyzer and micro-tubing reactor. Biores Technol. 2009;100:400–5.
Kim SS, Kim J, Park YH, Park YK. Pyrolysis kinetics and decomposition characteristics of pine trees. Biores Technol. 2010;101:9797–802.
Wongsiriamnuay T, Tippayawong N. Non-isothermal pyrolysis characteristics of giant sensitive plants using thermogravimetric analysis. Biores Technol. 2010;101:5638–44.
Sutcu H. Pyrolysis of Pragmites australis and characterization of liquid and solid products. J Indust Eng Chem. 2008;14:573–7.
Parikh J, Channiwala SA, Ghosal GK. A correlation for calculating elemental composition from proximate analysis of biomass materials. Fuel. 2007;86:1710–9.
Jiang G, Nowakowski DJ, Bridgwater AV. A systematic study of the kinetics of lignin pyrolysis. Thermoch Acta. 2010;498:61–6.
Iredale PJ, Hatt BW, Overend RP, Milne TA, Mudge LK, Editors, fundamentals of thermochemical biomass conversion. Amsterdam: Elsevier; 1985. p. 143–155.
Di Blasi C, Lanzetta M. Intrinsic kinetics of isothermal xylan degradation in inert atmosphere. J Anal Appl Pyrolysis. 1997;40–41:287–303.
Ferdous D, Dalai AK, Bej SK, Thring RW. Pyrolysis of lignins: experimental and kinetics studies. Energy Fuels. 2002;16:1405–12.
Wang J, Wang G, Zhang M, Chen M, Li D, Min F, Chen M, Zhang S, Ren Z, Yan Y. A comparative study of thermolysis characteristics and kinetics of seaweeds and fir wood. Proc Biochem. 2006;41:1883–6.
Jiang H, Wang J, Wu S, Wang B, Wang Z. Pyrolysis kinetics of phenol-formaldehyde resin by non-isothermal thermogravimetry. Carbon. 2010;48:352–8.
Li D, Chen L, Zhao J, Zhang X, Wang Q, Wang H, Ye N. Evaluation of the pyrolytic and kinetic characteristics of Enteromorpha prolifera as a source of renewable bio-fuel from the Yellow Sea of China. Chem Eng Res Desi. 2010;88:647–52.
Wongsiriamnuay T, Tippayawong N. Thermogravimetric analysis of giant sensitive plants under air atmosphere. Biores Technol. 2010;101:9314–20.
Cuña Suárez A, Tancredi N, César C, Pinheiro P, Irene M. Thermal analysis of the combustion of charcoals from Eucalyptus dunnii obtained at different pyrolysis temperatures. J Therm Anal Calorim. 2010;100:1051–4.
Gotor FJ, Criado JM, Málek J, Koga N. Kinetic analysis of solid-state reactions: the universality of master plots for analyzing isothermal and nonisothermal experiments. J Phys Chem A. 2000;104:10777–82.
Pérez-Maqueda LA, Criado JM, Gotor FJ, Málek J. Advantages of combined kinetic analysis of experimental data obtained under any heating profile. J Phys Chem A. 2002;106:2862–8.
Wang XB, Si JP, Tan HZ, Niu YQ, Xu C, Xu TM. Kinetics investigation on the combustion of waste capsicum stalks in Western China using thermogravimetric analysis. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1556-z.
Zou S, Wu Y, Yang M, Li C, Tong J. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Biores Technol. 2010;101:359–65.
Orfao JJM, Antunes FJA, Figueiredo JL. Pyrolysis kinetics of lignocellulosic materials-three independent reactions model. Fuel. 1999;78:349–58.
Rao TR, Sharma A. Pyrolysis rates of biomass materials. Energy. 1998;23:973–8.
López FA, Mercê ALR, Alguacil FJ, López-Delgado A. A kinetic study on the thermal behaviour of chitosan. J Therm Anal Calorim. 2008;91:633–9.
Peng WM, Wu QY, Tu PG, Zhao NM. Pyrolytic characteristics of microalgae as renewable energy source determined by thermogravimetric analysis. Biores Technol. 2001;80:1–7.
Kumar A, Wang L, Dzenis YA, Jones DD, Hanna MA. Thermogravimetric characterization of corn stover as gasification and pyrolysis feedstock. Biom Bioen. 2008;32:460–7.
Li D, Chen L, Yi X, Zhang X, Ye N. Pyrolysis characteristics and kinetics of two brown algae and sodium alginate. Biores Technol. 2010;101:7131–6.
Sathitsuksanoh N, Zhu ZG, Templeton N, Rollin J, Harvey S, Zhang YHP. Saccharification of a potential bioenergy crop, Phragmites australis (Common Reed), by lignocellulose fractionation followed by enzymatic hydrolysis at decreased cellulase loadings. Indus Eng Chem Res. 2009;48(13):6441–7.
Aboulkas A, Harfi KE, Nadifiyine M, Bouadili AE. Thermogravimetric characteristics and kinetic of co-pyrolysis of olive residue with high density polyethylene. J Therm Anal Calorim. 2008;91:737–43.
Açıkalın K. Pyrolytic characteristics and kinetics of pistachio shell by thermogravimetric analysis. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1714-3.
Aboulkas A, Harfi KE, Bouadili AE, Nadifiyine M. Study on the pyrolysis of moroccan oil shale with poly (ethylene terephthalate). J Therm Anal Calorim. 2009;100:323–30.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (21076117), and CAS International Partnership Program “Typical environment processes and their effects on resources,” and Outstanding young scholar fellowship of Shandong Province (JQ200914), and the Open Funding from the State Key Laboratory of Crop Biology in Shandong Agricultural University (2010KF06 and 2011KF14), and Projects of Shandong Province Higher Educational Science and Technology Program (J09LC22 and J10LC15), and the Open Funding from the Key Laboratory of Experimental Marine Biology, Institute of Oceanology (Kf201016), and Open Project Program of the Key Laboratory of Marine Bio-resources Sustainable Utilization (LMB101004), SCSIO, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. Yan and C. Zhang contributed equally as first authors.
Rights and permissions
About this article
Cite this article
Zhao, H., Yan, H., Zhang, C. et al. Thermogravimetry study of pyrolytic characteristics and kinetics of the giant wetland plant Phragmites australis . J Therm Anal Calorim 110, 611–617 (2012). https://doi.org/10.1007/s10973-011-2018-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2018-3